
|
BMe Kutatói pályázat |

|

BME-ABÉT F-Labor
1111 Budafoki street 6-8
FE staircase groundfloor 1.
Tel.: 463-2693
http://f-labor.mkt.bme.hu
Faculty of Chemical Technology and Biotechnology
Department of Applied Biotechnology and Food Science/Fermentation Pilot Plant Laboratory
F-Labor
Short introduction of the research area
The research in F-labor focuses on industrial applications (fermentations) of microorganisms, that stands in the center of biotechnological operations. In these microbial fermentations (carried out by bacteria, yeasts and other fungi, and viruses) lots of valuable (market)products forme during cell growth. Examination and optimization of these product formation is the main task of our research group. During development of a fermentation technology the primary goal is to quantify and satisfy the demands of the microbe in order to obtain maximal productivity. For this, our research usually starts in lab scale with selection of the appropriate producer strain, than follows with optimisation (1. nutrient requirements (carbon, nitrogen sources, trace demand, vitamin demand etc), 2. condition parameters: pH, temperature, aeration, fermentation technique etc.). Finally, fermentation in different (pilot) scales can improve the reliablilty of the developed process, that serves as basis for technology transfer.
that stands in the center of biotechnological operations. In these microbial fermentations (carried out by bacteria, yeasts and other fungi, and viruses) lots of valuable (market)products forme during cell growth. Examination and optimization of these product formation is the main task of our research group. During development of a fermentation technology the primary goal is to quantify and satisfy the demands of the microbe in order to obtain maximal productivity. For this, our research usually starts in lab scale with selection of the appropriate producer strain, than follows with optimisation (1. nutrient requirements (carbon, nitrogen sources, trace demand, vitamin demand etc), 2. condition parameters: pH, temperature, aeration, fermentation technique etc.). Finally, fermentation in different (pilot) scales can improve the reliablilty of the developed process, that serves as basis for technology transfer.
Short introduction of the research place
The name "F-Labor" disclose several things (especially in hungarian), like:

Building F |

Fermentation |

Downstream and proteinpurification |
 Pilot scale |
The Fermentation Pilot Plant research group has been founded more then 25 years ago, and its recent infrastructure has been also working in its present form since a decade. Our infrastructure is used for industrial-coworkers as well as educational and research purposes. The pilot scale facilities generated our potential to join the first Hungarian Biorefinery project, which examines the fermentative production possibilities of lactic acid.
The history and detailed description of the research
It was a strategic question in the 80's in Hungary, wheather the yet operating biotechnological plants should be improved or be weared out. According to strong efforts of The Bioengineering Workinggroup of HAS they have been improved, and to fullfill the rising pilot scale research demand was our Fermentation group founded at BUTE Faculty of Chemical Engineering under the then existing Department of Agricultural Chemical Technology. The frequent industrial interests with the scientific and technological improvements resulted a modern fermentation pilot plant, joining such projects like the antibiotics production at Biogal Pharma, or B12 fermentation in Richter Pharma or lysine (amino acid) production technology in Kaba.
When biotechnology is divided into groups according to the widely accepted colorcodes, than the above mentioned researches are from the field of Red (Health and Pharma) Biotechnology. Although our research group had already cooperations also on the field of Green (feed and food, and agriculture) Biotechnology (for example to produce preparation against the Fire blight of the apples and its relates), recently researches on the filed of White Biotechnology are the most dominant, partly 1,3-propandiol enzymatic production, and on the other hand fermentative lactic acid production. Both researches are involved in the BUTE Research University project as topics of Researches for White Biotechnology methodes under the Biotechnology-Health and Environment protection priority research area.
The aim of te white biotechnology branch is to "whiten" or clean the chemical industry, that is to convert it into environmentally neutral form. (The goals are partly the same than in green chemistry but the tools are significantly different.)
The structure of the Chemical industry is strongly hierarchic with petroleum on the top as single energy and raw material source. With its refining can the first basic molecules be obtained, from which the chemical reactions starts in the industry. On the analogy of these refineries was the term "biorefinery" introduced, that meant first only the conversion of some renewables (like starch) into products, but recently the 2nd and 3rd generation of them are under planning and construction, which can flexibilly adapt to different rawmaterials and market demands. Thus it results in a technological matrix instead of the former hierarchy.
From the renewable (plant origin) rawmaterials Biorefineries produce basic moleculs which are called "platfrom-forming" chemicals, which are equally valuable for the classic chemical industry. 1,3-propanediol and lactic acid are both such platform chemicals. While the former can be used as solvent or as anti-freezing agent but mostly as a monomer for the plastic industry, lactic acid is widely used in food industry (preservative), cosmetic (hydrating agent) and pharma (biodegradable surgeon cord) purposes. From Poli-lactic acid (PLA) can biodegradable polymer be produced, thus it is one of teh target moleculs for packaging industry.
The goal of the reasearch, questions to be answered
The biological production of 1,3-propanediol was realized paralell with our researches: the fermentaion plant of Tate & Lyle started in 2006 as a result of an international cooperation, in which de novo recombinant Escherichia coli produces 40.000t/yr 1,3-propanediol. In case of microbial fermentations a given part of the raw material is converted into (cell)biomass, and decreases the product formation, and furthermore usually some metabolites are also arising causing further loss of the raw material. Considering these, we developed a coenzyme linked process in labscale, in which the byproduct of biodiesel (namely the glycerol, which is a renewable in this context) will be converted into 1,3-propanediol and oxidized coenzyme (NAD+). This oxidized coenzyme will simultaneously be backreduced in a revert reaction meanwhile an other molecule of glycerol will be oxydized into 1,3-dihydroxyacetone (DHA), which is the active agent of self-tanning preparations in the cosmetic industry. Through this way in one simple step a renewable waste can be converted into two valuable industrial products, without byproducts.
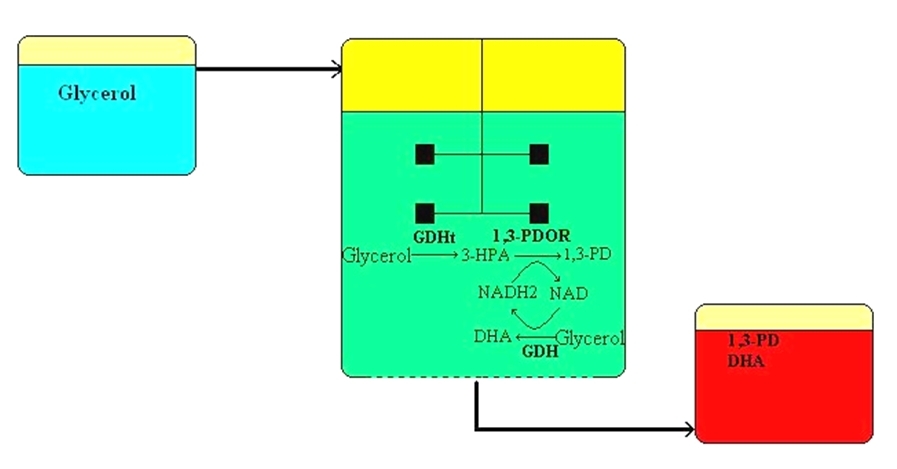
During the research we pointed out the enzyme production, since they are not available commercially. Beside with 1,3-propanediol producer strains existing in nature we started to develop our own recombinant E. coli for more effective enzyme production purposes. Several questions have already been answered during our research so far (for Example: Can such a system operated? Yes, it can. Are the enzyems capable for using in several cycles? Yes, they are. etc.), but several important questions are still open, like: Can batch operation of this system be turned into continuous mode? What are the common optimal pH and temperature values and enzyme ratio of the three keyenzymes? Is it possible to scale up the process? ,etc.
The first milestone of the lactic acid research was reached, and our results were summerized in Kata Hetényi's PhD dissertation (i.e. thesis). During these we observed, which mesophillic microbe on which agricultural rawmaterials what kind of nutrient supplementation need for effective L-lactic acid production, and furthermore what the technical paramters of these are. Some thermotolrant producer strain were also selected, but further examination od these is nesseceray to overcome their productinhibition by lactic acid, their pH controlling challenges and their scale up, which researches are running now exactly.
Methods
For the enzymatic bioconversion of 1,3-propanediol a special Clostridium butyricum strain was applied. Its speciality is, that while other 1,3-propandiol (and its keyenzymes) producer have a B12 dependent glycerol-dehydratase (GDHt) which goes under suicid inactivation with its coenzyme, the GDHt of the applied C. butyricum is B12-independent. At the same time, this microbe needs strict anaerobic conditions (full air displace) which makes the enzyme production more difficult. As other bacteria, this strain is also best cultivated on complex media, which is technologically not advantageous, because of its high and costly organic nitrogen-source content (like: tryptone, yeast exract) which remain mostly intact causing difficulties during enzyme recovery, and can contaminate the product. For this reasons, we make great efforts to determine the required minimal but sufficient components and their quantity.
The key enzymes are formed intracellulary (inside the cells), thus the culture is sonicated after fermentation, and celldebris is removed with centrifugation. In the obtained supernatant solution there presents the whole protein (and enyzme) content of the cells, and the key enzymes should be purified from this protein matrix. We also examined, how far should be the enzymes purified to keep the process cost effective while even having the nessesary biocatalytic activity. The obtained enzymes are quite sensitive especially against the oxygen, thus their stabilizing is a crucial point for any application. We have good results with covalent immobilisation in this respect. To immobilise the enzymes we use chitosan (produced from fishing wastes (chitin)) for manifacturing polymer beads with glutar-aldehyde, which bind enzymes to the beads. After immobilisation of the key enzymes this way -according to our results- the enzymes have significantly higher stability.
It is an important part of this research to determine the activtity of the enzymes. This task is quite difficult because of the complex background matrix, even in case of soluble enzymes.In case of immobilised enzymes the analytical process is under investigation recently.
From the begining of the research the enzyme source is the above mentioned C. butyricum, but this natural strain has limited possibility (enzyme recovery is limited, byproduct formation problems, anaerobic demand etc.), thus we started to clone the key enyzmes into a recombinant bacterium. The possible succes of this way will give a new impetus for the examination of the enzymatic system.
In case of lactic acid the applied methods were summerized at several publications, thus we mention them only briefly here: 1) we selected a Lactobacillus strain, and scaled up on a laboratory media 2) this was reproduced in real industrially applicable media, too (primerly on wheat floor basis, later on basis of sweet sorghum broth, and determined in both cases the nutrient supplemenetation with statistical experimental design), 3) then we solved the pH controlling problems 4) and finally we set up and applied a kinetic model for determination of the optimal inoculation time during starch hydrolysis (in wheat flour) to obtain a combined hydrolysis and fermentation technique.
Since on both raw materials we found that a minimal yeast extract demand is essential, - according to the Biorefinery concept - we examined the possibility of the in house yeast extract production via yeast fermentation on different wastes of sweet sorghum (i.e. cellulose or hemicellulose fraction of bagasse). To decide, wheather sweet sorghum broth or pentose or hexose fraction of the bagasse would be economically more feasible, we used flowsheeting for technology simulation. This indicated that sweet sorghum broth is the most cost effective way for an in house yeast extract production.
Recent results
During the enzymatic 1,3-propanediol production we have several successful glycerol-propanediol bioconversions in a membrane reactor (enzymes immobilized behind an ultrafilter membrane) with batch operation. After discharging the whole broth throuh the membrane and refilling the reactor with fresh glycerol solution it was verified, that even the soluble enzymes are capable for multicycle usage. Since the enzyme solution was not purified, in this cases dihydroxyaceton (DHA) was phosphorylated and partly converted by the glycolitic enzymes into byproducts (like butyric acid, acetic acid, lacticacid).
Expected impact, further researches
We created patent submissions from both researches (1,3-propanediol and lactic acid P 05 00961 and P 09 00193 respectively) and two summa cum laude PhD dissertations with summarized impact factor of 5.84 for the international publications. The reserch group is ready for technology transfer in both fields for potential investors, although in both cases we have to do some further investigations. For exampe in case of lactic acid the literture shows several efficient fermentation technics in laboratory scale, but they were still not scaled up or used in the industry, and we have the possibility in our pilot laboratory to answer, why they did not spread.
In case of PD, we already have results on the filed of enzyme stabilization and their simultaneous application, but with further optimisation we expect to reach additional cost-effectivity, and thus increse the competitivness of our process.
Own publications, references, linkcollection
Connected own publications:
(5 outstanding publication from the last 5 years)
Hetényi, K.; Németh, Á.; Sevella, B., Investigation and modeling of lactic acid fermentation on wheat starch via SSF, CHF and SHF technology. Periodica Polytechnica Chemical Engineering 2011, 55(1), 11-16. (online)
Hetényi, K.; Németh, Á.; Sevella, B., Role of pH-regulation in lactic acid fermentation: second steps in a process improvement. Chemical Engineering and Processing: Process Intensification 2011, 50, 293-299. (online)
Hetényi, K., Németh, Áron., Sevella, Béla, Use of sweet sorghum juice for lactic acid fermentation: preliminary steps in a process optimization. Journal of Chemical Technology and Biotechnology 2010, 85, 872-877. (online)
Balássy, A., Németh, Á., és Sevella, B, Immobilized enzymes availability for glycerol -1,3-propanediol bioconversion. Hungarian Journal of Industrial Chemistry 2009, 37, 83-88.(online)
Németh, Á.; Sevella, B., Development of a New Bioprocess for Production of 1,3-propanediol I.: Modeling of Glycerol Bioconversion to 1,3-propanediol with Klebsiella pneumoniae Enzymes. Applied Biochemistry and Biotechnology 2008, 144, (1), 47-58. (online)
(5 earlier publications)
Balássy, A., Németh, Á., and Sevella, B In New Enzymatical Process for Anaerobic Utilization of Glycerol, ChemPor 2008 10th International Chemical and Biological Conference, Braga, Portugalia, 4-6. September, 2008;
Hetényi, K., Németh, Á. and Sevella, B., Researches on renewable resources at BUTE ABFS F-Labor, Fifth Croatian Professional and Scientific Conference on Biotechnology with International Participation, Stubicke Toplice, 2007;
Németh, Á.; Sevella, B., Kutatások a biodízel melléktermékének hasznosítására. Magyar Kémiai Folyóirat 2007, 113, (2), 58-61. (online)
Németh, Á.; and Sevella, B. Forschungen für enzymatische Herstellungen der industriellen wertvollen Glyzerin-Derivate, 17. Frühlingsakademie von Institute für Ingenieurweiterbildung der Technische und Wirtschaftwissenschaftliche Universitaet Budapest, Balatonfüred, 6th may in, 2005
Németh, Á.; Kupcsulik, B.; Sevella, B., 1,3-Propanediol oxidoreductase production with Klebsiella pneumoniae DSM2026. World Journal of Microbiology and Biotechnology 2003, 19, (7), 659-663. (online)
Linkcollection
Bioengineering Working Grouop of HAS
Lactic acid from sweet sorghum
E-Vegyérték-electronic lecture notes
Introduction of participants

László Nyeste
retired professor |

|
|

|
|

Áron Németh senior lecturer |
|
|

|
|

Mrs Vargyas
cleaning referee |
|
|