 |
BMe Research Grant |

|
BME, Oláh György Doktoral School
Department of Inorganic and Analytical Chemistry
Supervisor: Dr. Tamás Veszprémi
Molecular tailoring: Chemical reaction design by bulky groups
Introducing the research area
During my work I investigate the special effect of bulky groups in chemical reactions using computational chemical methods. My research gives a new approach to design hitherto unknown chemical reactions and also to modify the rate of already used ones.
Brief introduction of the research place
Studying low coordinated carbon and silicon compounds with theoretical methods has a long history at the Department of Inorganic and Analytical Chemistry. Our several international collaborations and publications in leading journals indicate the high level of our research.
History and context of the research
An old wisdom in chemistry says that reactions can be slown down or even stopped by large, inert groups situated near the reaction centers. Then these steric or in other name bulky groups can simply hinder the reaction centers getting close to each other and block the reaction (Fig. 1). This approach has become important in the synthesis of reactive systems, where the goal is the lack of reaction, thus some really surprising results occurred in the last decades.
.
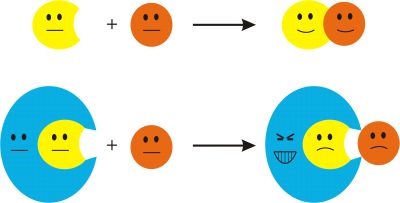
Fig. 1: Effect of bulky groups
One example is the disproof of the double bond rule. The double bond rule claims that multiple bonds can only exist between carbon, nitrogen and oxygen atoms. Chemists believed that double bonds between other elements could be too weak to be stable. Therefore, the first synthesis of the stable Si = Si double bond was groundbreaking [1, 2]. After that several interesting discoveries occurred in this field [3, 4, 5].
Another surprising result was the preparation of divalent carbon and silicon compounds (carbene and silylene) [6, 7]. Carbon and silicon are used to form four chemical bonds. However, based on their position in the periodic table, they may be able to form only two bonds, which has been shown. Moreover, a formally zero bonded carbon compound has been recently reported [8].
Beyond chemical curiosity, this field became widely studied after these compounds proved to be potentially excellent catalysts. For example, certain carbenes can activate hydrogen better than platinum [9]. As a result, several transition metal based technology can be replaced with environmental friendly processes. Therefore, the investigation and better understanding of the reactions of carbenes and silylenes are essential. The situation is described expressively by the fact that the number of papers published about carbenes and the recent “star”, graphene is similar according to Web of Science.
The research goal, open questions
As we have seen, bulky groups play decisive role in the stabilization of reactive compounds hindering their decomposition. For this reason they also influence the reactions that we want to realize with these reactive centers on purpose. Theoretical studies have not dealt with real, experimentally applied bulky groups because of their vast computational cost. Therefore, model compounds are used to study where bulky groups are replaced with hydrogen atoms, so their effect is neglected. However, thanks to high performance computing and to the development of theory, now it is possible to investigate real systems with bulky groups.
During my work I focused on reactions which cannot be explained by model compounds. I had found several anomalous reactions in the literature which suggested that in some cases bulky groups may have special features beyond their well-known hindering property. It was my task to understand these special features, solve these anomalies and complement their theory. My work can be sorted into two topics.
After the disclaim of the double bond rule several surprising structures were synthesized with high yield by only changing bulky groups [10, 11, 12, 13]. According to the calculations on model compounds, several energetically similar structures can be possible. Therefore, the question arises how bulky groups can have such a precise and selective reaction controlling ability.
White phosphorus (P4) is an important starting point of several pesticides and insecticides. The activation of P4 can be done by expensive and poisonous heavy metal complexes with recent technologies. The exchange of those complexes to cheap and environmental friendly compounds would be useful. The reaction of P4 is known with multifarious carbenes [14, 15, 16] but with only one silylene [17] the cause of which is still a mystery.
Methodology
During my work, I apply state-of-the-art methods of theoretical chemistry and physics (ab initio, post-HF methods, density functional theory, QTAIM) using high performance computers. The main question is how to make compatible classic chemical concepts such as chemical bond, stable molecule, chemical reaction with the formulas of modern physical theories.
One of its most important parts is the so-called Potential Energy (Hyper)Surface (PES) which can be derived from Schrödinger-equation with the Born-Oppenheimer approximation. It states that an energy value can be associated to every configuration of atomic nuclei which determines a 3N-6 dimension surface (N is the number of atomic nuclei). Chemical reactions can be equivalent to migration on this surface. Special one dimensional sections of PES are well-known for chemists which can be connected to the concept of reaction coordinate (Fig. 2). Reaction coordinate (q) is a geometric parameter which measures the progress along the reaction path. The maximum of the curve is the transition state that provides the energy barrier which needs to pass that the reaction proceeds.
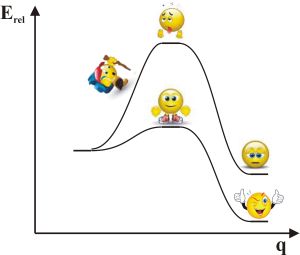
Fig. 2: Reaction coordinate
The transition state theory of reaction kinetics connects this barrier with the rate of chemical reactions. The transition state on the PES is the pass between two valleys, that is, a saddle point. During the exploration of the reaction mechanism we try to locate these saddle points in our simulations. The problem is that there is no exact algorithm to find these points. Therefore, in spite of useful approximations, chemical intuition and serious experience in the field and also good luck is required to gain these results within reasonable time.
The classic theory of bulky groups can be simply displayed in the one dimensional sections of the PES (Fig. 2). Owing to the effect of bulky groups the transition state increases slowing down chemical reactions.
Results
The formation of reactive silicon compounds
pointed out that the precise selectivity effect of the bulky groups did not stem from the selective increasing of the activation barrier during the reaction but from the change of the shape of the PES (Fig. 3) [T1]. The experimentally applied bulky groups destabilize all competitive structures which means that these structures are eliminated as minima on the PES. As a result, technically only one reaction channel remains which leads to the product. In addition, the bulky group can cover the product so well that they inhibit the decay of the reactive structure.
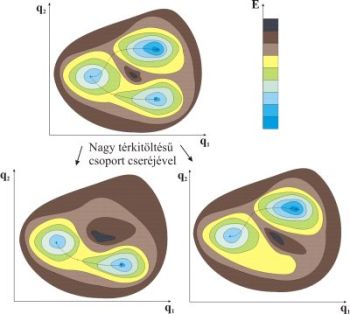
Fig. 3: The potential effect of bulky groups on reaction
Based on our results, we designed bulky groups for the synthesis of hexasilabenzene (Fig. 4) which is regarded as the “holy grail” of silicon chemistry. The experimental work has been taken immediately by two groups (Prof. R. West, University of Wisconsin, USA and Prof. K. Tamao, RIKEN, Japan).
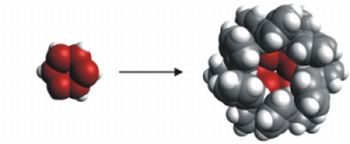
Fig. 4: Hexasilabenzene design
Activation of white phosphorus by silylenes
I pointed out that this unique reaction cannot be explained by model compounds [T2, T3]. The study of the effect of the bulky groups suggests [T4] that in one case the rate determining activation barrier surprisingly decreases, while in other cases these barriers increase as expected (Fig. 5). The bulky group promotes the reaction in this case which has been never demonstrated before!
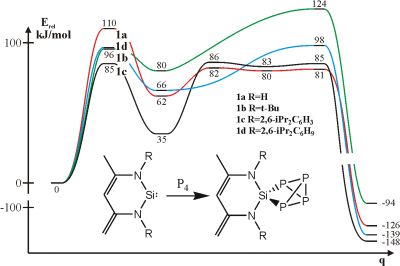
Fig. 5: Activation of white phosphorus by silylene
The solution to this peculiar effect is the nonbonding interaction which can be shown in the van der Waals representation of the molecule (Fig. 6). Based on this, an explanation can be set up parallel to enzyme catalysis. Red arrows indicate the steric hindrance between the yellow phosphorus atoms and the bulky groups. In case of the anomalous substituent (1c) there are no red arrows showing that the four phosphorus atoms perfectly fits in the hollow created by the bulky groups, and there is only favorable nonbonding interaction between the phosphorus atoms and the bulky groups.
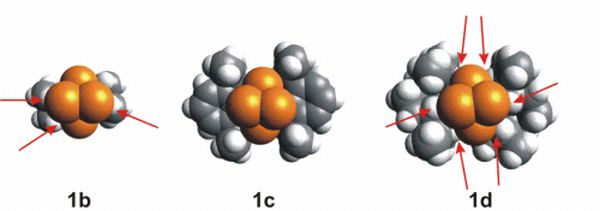
Fig. 7: Van der Waals representation of silylene with different bulky groups. Red arrows indicate steric hindrance
The effect of enzymes can be explained similarly where shape selectivity and specific nonbonding interactions have decisive role. This result can also explain the reaction of carbenes because all reacting carbenes have been synthetized using the same special bulky group.
Fig. 7: The principle of modification of bulky groups
With this principle in mind (Fig. 7) we managed to design a silylene where the decrease of the activation barrier is more significant [T5]. The experimental work is done by Prof. R. West (University of Wisconsin) and his colleagues.
Expected impact and further research
During my work, I pointed out how bulky groups can selectively control the synthesis of reactive silicon compounds or even modify the rate of reaction. These results open up new possibilities; therefore we have published them in leading international journals. Our specific results are based on new general principles which can be widely applied in chemistry. Guided by the principle and using the design methods several hitherto unknown reactions can be realized or we can speed up or slow down others on demand.
As recognition of my results I was awarded by the German North Rhine Westphalia Young Scientist Award in chemistry. Hungarians or even Eastern Europeans have never been awarded with this highly prestigious prize before.
In the future I plan to focus on applying our recent results in the widest possible range [T6].
Publications, references, links
Publications
[T1] T. Szilvási, T. Veszprémi: Molecular-tailoring: Reaction path control with bulky substituents, Organometallics, 31, 3207 (2012)
[T2] K. Nyíri, T. Szilvási, T. Veszprémi: The mechanism and energetics of insertion reactions of silylenes, Dalton Trans. 39, 9347 (2010)
[T3] T. Szilvási, K. Nyíri, T. Veszprémi: On the unique insertion mechanisms of β-diketiminato-silylene, Organometallics, 30, 5344 (2011)
[T4] T. Szilvási, T. Veszprémi: On the mechanism of the reaction of white phosphorus with silylenes, Dalton Trans. 40, 7193 (2011)
[T5] T. Szilvási, R. West, T. Veszprémi: Molecular-tailoring: Internal catalytic effect of bulky substituents, publikációra előkészítve
[T6] T. Szilvási, T. Veszprémi: Molecular-tailoring: Internal catalytic effect of bulky NHC ligands in Suzuki-Miyaura coupling: Revised theory of bulky groups, publikációra előkészítve
Links
References
[1] R. West, M. J. Fink, J. Michl: Tetramesityldisilene, a stable compound containing a silicon-Silicon double bond, Science, 214, 1343 (1981)
[2] R. West: Isolable Compounds Containing a Silicon-Silicon Double Bond, Science, 225, 4667 (1984)
[3] L. Gagliardi, B. O. Roos: Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond, Nature, 433, 848 (2005)
[4] T. Nguyen, A. D. Sutton, M. Brynda, J. C. Fettinger, G. J. Long, P. P. Power: Synthesis of a stable compound with fivefold bonding between two chromium(I) centers, Science, 310, 844 (2005)
[5] S. Shaik, D. Danovich, W. Wu, P. Su, H. S. Rzepa, P. C. Hiberty: Quadruple bonding in C2 and analogous eight-valence electron species, Nature Chemistry, 4, 195 (2012)
[6] A. J. Arduengo III, R. L. Harlow, M. Kline: A stable crystalline carbene, J. Am. Chem. Soc. 113, 361 (1991)
[7] M. Denk, R. Lennon, R. Hayashi, R. West, A. V. Belyakov, H. P. Verne, A. Haaland, M. Wagner, N. Metzler: Synthesis and structure of a stable silylene, J. Am. Chem. Soc, 116, 2691 (1994)
[8] C. A. Dyker, V. Lavallo, B. Donnadieu, G. Bertrand: Synthesis of an extremely bent acyclic allene (a “carbodicarbene”): a strong donor ligand, Angew. Chem. Int. Ed. 47, 17, 3206 (2008)
[9] G. D. Frey, V. Lavallo, B. Donnadieu, W. W. Schoeller, G. Bertrand: Facile splitting of hydrogen and ammonia by nucleophilic activation at a single carbon center, Science, 316, 439 (2007)
[10] A. Sekiguchi, T. Yatabe, C. Kabuto, H. Sakurai: The "missing" hexasilaprismane: synthesis, X-ray analysis, and photochemical reactions, J. Am. Chem. Soc. 115, 5853 (1993)
[11] A. Sekiguchi, R. Kinjo, M. Ichinohe: A stable compound containing a silicon-silicon triple bond, Science, 305, 1755 (2004)
[12] K. Abersfelder, A. J. P. White, H. S. Rzepa, D. Scheschkewitz: A tricyclic aromatic isomer of hexasilabenzene, Science, 327, 564 (2010)
[13] K. Suzuki, T. Matsuo, D. Hashizume, H. Fueno, K. Tanaka, K. Tamao: A planar rhombic charge-separated tetrasilacyclobutadiene, Science, 331, 1306 (2011)
[14] J. D. Masuda, W. W. Schoeller, B. Donnadieu, G. Bertrand: Carbene activation of P4 and subsequent derivatization, Angew. Chem. Int. Ed. 46, 7052 (2007)
[15] J. D. Masuda, W. W. Schoeller, B. Donnadieu, G. Bertrand: NHC-mediated aggregation of P4: Isolation of a P12 cluster, J. Am. Chem. Soc. 129, 14180 (2007)
[16] O. Back, B. Donnadieu, P. Parameswaran, G. Frenking, G. Bertrand: Isolation of crystalline carbene-stabilized P2-radical cations and P2-dications, Nature Chemistry, 2, 369 (2010)
[17] Y. Xiong, S. Yao, M. Brym, M. Driess: Consecutive insertion of a silylene into the P4 tetrahedron: Facile access to strained SiP4 and Si2P4 cage compounds, Angew. Chem. Int. Ed. 46, 4511 (2007)
