 |
BMe Research Grant |

|
George A. Olah Doctoral School in Chemistry and Chemical Technology
Institute of Isotopes, Centre for Energy Research, Hungarian Academy of Sciences, Radiation Chemistry Laboratory
Supervisors: Dr. Erzsébet Takács, Dr. László Wojnárovits
Water treatment by ionizing radiation
Introducing the research area
One of the most important environmental challenges is the constant supply of good quality drinking water. Soil and drinking water are contaminated because of the rapid growth of the population and the large-scale development of agriculture and industry. Organic pollution (especially drugs and their metabolites) is rapidly growing. Their natural degradation requires a long time and they can accumulate in the food chain. New procedures are studied nowadays for the demolition of these compounds in aqueous media (AOP).
My research field aims at the degradation of drug molecules in dilute aqueous solutions, induced by ionizing radiation.
Brief introduction of the research place
The experiments were carried out at the Institute for Isotope Research, Centre for Energy Research, Hungarian Academy of Sciences, Radiation Chemistry Laboratory.
The Laboratory research activities address two main areas: environmental protection and irradiation of polymers.
The Laboratory deals with the study of kinetics and mechanism of high energy radiation induced reactions such as polymerization, grafting (synthetic and natural polymers, e.g. cellulose), polymer degradation, degradation of organic pollutants in water (wastewater treatment) to determine the parameters for the radiation induced synthesis of polymers with special properties. The aim is to determine the sensitivity of materials to radiation and to optimize the parameters for technological applications of ionizing radiation.
History and context of the research
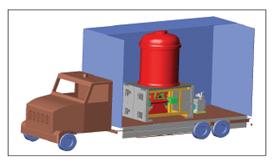
Ionizing radiation was first used in the early 80’s in East Germany for environment-protection purposes: drinking water was disinfectioned by 60Co gamma radiation. The first devices with electron accelerator were built in the USA. They were used in the pre-treatment process of communal wastewater cleaning, the electron accelerator was built in a lorry [1].
There are several pilot wastewater cleaning plants in Russia as well, where the pre-treatment of wastewater is done by gamma radiation. These pilot plants were built near the oil refineries and alcohol-distillers [2].
The International Atomic Energy Agency supported the South-Korean pilot (in 1998) and the industrial scaled (in 2005) electron accelerator in a wastewater cleaning plant. (See the flow chart below.)
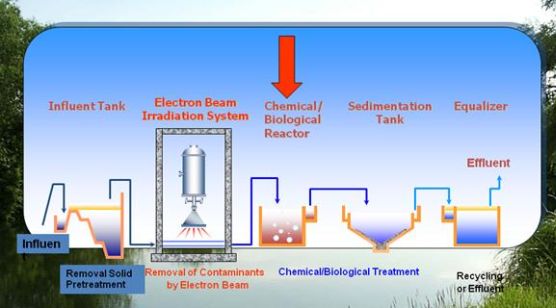
Fig. 1: Process flow diagram
The experimental results coming from the South-Korean wastewater plant are shown in Fig. 2. The reduction of chemical oxygen demand (COD) of the wastewater treated by a relatively small dose of 2 kGy has the same rate after 6 hours of treatment as that of the untreated wastewater after 16 hours.
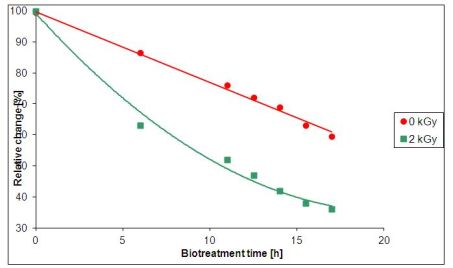
Fig. 2: Dependence of COD on the time of irradiation [3]
The research goal, open questions
No real industrial breakthrough has been achieved so far partly in consequence of lack of knowledge. The goal of my research is to study the chemical background of the wastewater treatment technology, to determine the technological parameters, to supply basic data of drug degradation in aqueous solutions, to identify the intermediate and final products and to determine the chemical oxygen demand and total organic carbon content.
The radiolysis of water gives a distribution of transient and stable products according to the equation (1).
The values in brackets are yields, the so-called G-values in mmol J-1 units. In order to study the reactions of the three reactive intermediates separately (·OH, e-aq and H·) different additives and techniques have been applied [4]. In N2O saturated solutions due to the transformation in (2) the reacting radicals and their yields are: hydroxyl radical 0.55 µmol J-1, hydrogen atom 0.06 µmol J-1. The reaction of hydrated electron with the molecules is studied in N2 saturated 5 vol% tert-butanol containing solution. In such solutions the ·OH radicals react with tert-butanol forming rather
inactive tert-butanol
radicals (3).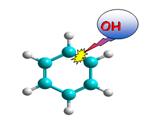
In air saturated solution due to the low solute concentration applied, H· and e-aq practically entirely react with O2, therefore ·OH radicals and the HO2·/O2-· pair (pKa = 4.8) are the possible reactive intermediates. They have low reactivity with aromatic compounds [5].
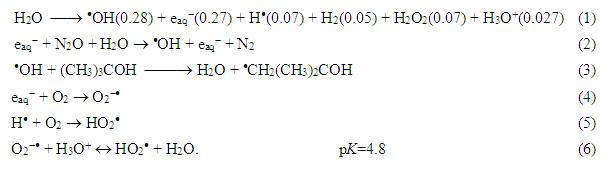
Methodology
The degradation of the molecules was studied by using two methods: gamma and pulse radiolysis. I carried out the gamma radiolysis experiments with + PBq (1600 TBq) activity of 60Co γ-source. The experiments were performed at 22 ± 3°C.
The pulse radiolysis is a unique tool for creating • OH radicals and other strong oxidants in water medium and for studying their reactions. In my present work, the creation and degradation of the intermediate product formed in the reaction of hydroxyl radical and diclofenac were studied. The solutions were bubbled in dinitrogenoxid. The technique uses short pulse of accelerated electrons. It triggers the development of reactive intermediates, radicals and ions.
The intermediates are studied using pulse radiolysis, while the degradation is followed by UV-VIS spectroscopy, separation by HPLC and products identification by diode array and MS-MS detection. The effects of dose, irradiation atmosphere and additives are investigated. The chemical oxygen demand, the total organic carbon content and the toxicity of the solutions are also studied.
The chemical oxygen demand (COD) means the amount of oxygen needed for the oxidation of dissolved and suspended organic matters in unit volume of water (mg / l). COD is one of the most important features of wastewater samples. COD value of the untreated and treated wastewater samples provides information about the extent of the degradation of organic matter that has taken place during the oxidation.
Determination of the total organic carbon (TOC) content is applied by different research areas. The TOC analysis is suitable for investigating the content of organic matter in wastewater and its chemical characterization.
It is essential that the treated water leaving the wastewater plant has low toxicity. Thus, studies have not only taken into account the degradation level of organic compounds (COD, TOC), but toxicity levels were also studied. The toxicity was investigated by using luminescent bacteria. The more the dose is the less luminescence can be observed. It indicates lower toxicity.
The HPLC (High Performance Liquid Chromatography) separation is based on the different physico-chemical properties of the moving components and the distribution of different phases.
Mass spectrometer was used to identify the components (MS). The identification based on the fragmentation of molecules and the determination of the mass of the formed ions
Results
I studied the following compounds: 2,4-dichlorophenoxi acid [H1], diclofenac [H2], paracetamol [H3] and 2,6-dichloroaniline [H4]. Here I review the diclofenac related experiments.
The pharmaceutical companies take into account the environmental toxicity when designing new molecules; from the different variants they try to choose the less toxic one. However, metabolites, degradation products may also be toxic for the fauna and flora. For instance, diclofenac (DCF), a non steroid type anti inflammatory drug, is a moderately toxic compound. During its degradation 2,6-dichloroaniline (DCA) forms which is much more toxic than the initial molecule. We study the radiation induced degradation of DCF and DCA with the purpose of eliminating these compounds from water.
The degradation was monitored by spectrophotometry. Fig. 3 shows that the degradation is proportional to the dose. It can be observed that DCF molecules can be easily degraded by radiation. The efficiency of degradation is the highest in the case of e-aq reaction and the smallest when irradiation is carried out in open air.
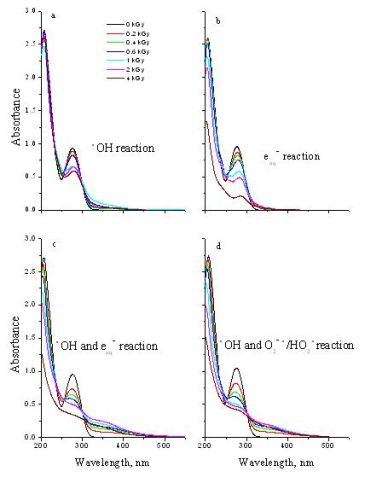
Fig. 3: Absorption spectra of 0.1 mM DCF solution irradiated with 0–4 kGy doses
Fig. 4 shows the chromatograms taken on 1 mM DCF solutions using the most characteristic wavelength from the datasets obtained by diode array detection. The insets show the absorption spectra at the maxima of the chromatographic peaks.
Four main products (P1, P2, P3, P4) were identified by Triple Quad LC / MS and using also authentic standard. The P1 product was identified as 5 hidroxydiclofenac, the P2 as 2,6-dichloro-anilin, the P3 as 4-chlorakridin and the P4 as kinoidal type of compound.
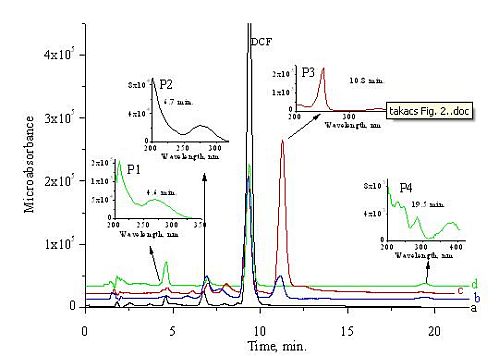
Fig. 4: HPLC chromatograms of 1 kGy irradiated 1 mM DCF solutions from the _OH reaction at 274 nm (a), from the e_aq reaction at 255 nm (b) from the _OH + e_aq reaction at 250 nm (c), and from the air saturated solution
The Chemical Oxygen Demand and Total Carbon Content measurements were carried out in 5×10-4 mol dm-3 mol dm-3 DCF solution; during the irradiation we applied a gentle air bubbling to avoid oxygen depletion. The COD values show a linear dependence on the dose up to 20 kGy; at this dose COD decreases by 78%. After this dose the curve levels off at a constant value which is about 10% of the starting COD. The dose dependence of TOC is similar to the dose dependence of COD, however, the TOC value at high doses decreases to zero.
DCF is a moderately toxic compound; -log EC50 (EC50, effective concentration for 50% luminescence reduction) is published as 4.03 [6]. We determined a value of 4.17 (DCF-Na) [H2]. In 0.1 mM DCF solution after 30 min incubation the starting luminescence intensity decreases by 87% compared to no DCF present. After ~1 kGy irradiation the toxicity remains practically unchanged (Fig. 4). By increasing the dose, the retardation of luminescence decreases indicating a decrease in toxicity. The luminescence intensity observed in solutions irradiated with low dose may show that some of the products are also toxic. For 2,6-dichloroaniline (2,6-DCA) -log EC50 of 4.97 is given [7] so about 8 times lower concentration than for DCF causes a 50% reduction in the luminescence yield. At 1 kGy in 0.1 mM solution the degradation of DCF molecules is practically complete, so there is no further source for 2,6-DCA formation [H4].
Fig. 5: Decrease of COD and TOC as a function of absorbed dose in air bubbled solution
In the reaction of hydroxyl radical and DCF molecules an intermediate product forms. Its maximum absorption is between 330 and 400 nm. The transient spectral change shows that the concentration of the intermediate product decreases in time (Fig. 6). According to the spectrum, these products are ciklohexadyenyl-type radicals.
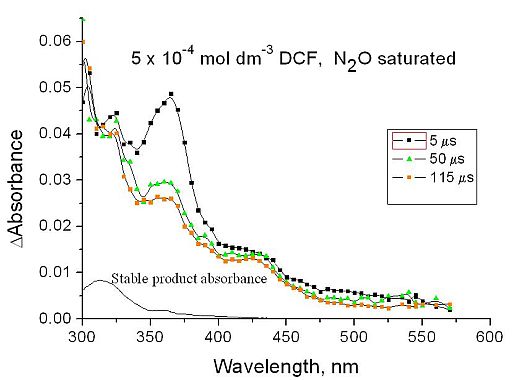
Fig. 6: Absorption spectrum obtained in pulse radiolysis of 0.5 mmol dm-3 DCF solutions
The recorded UV spectra, HPLC chromatograms and the transient spectra showed that diclofenac can effectively be degraded by ionizing radiation, in both oxidizing and reducing conditions. The way of degradation and the intermediate products can vary strongly in different conditions of treatment.
Expected impact and further research
Although the current chlorination-based water cleaning technology disinfects the water, the drinking water still contains chlorinated derivatives of organic matter (e.g. humic acids, hydrocarbons, drug molecules), which are harmful to human body. The example of diclofenac showed that organic compounds (such as environmentally hazardous drug molecules) can be easily degraded by using radiation technology, moreover, radiation technology can guarantee disinfection as well. Therefore, we recommend using radiation technology to treat wastewater polluted by bacteria and organic compounds (such as pharmaceuticals).
Publications, references, links
Publications
H1. Homlok, R., Takács, E., Wojnárovits, L.: Radiolytic degradation of 2,4-dichlorophenoxyacetic acid in dilute aqueous solution: pH dependence, J. Radioanal. Nucl. Chem., 2010, 284, 2, 415-419
H2. Homlok, R., Takács, E., Wojnárovits, L., Elimination of diclofenac from wastewater using irradiation technology, Chemosphere, 2011, 85, 4, 603-608
H3. Szabó, L., Tóth, T., Homlok, R., Takács, E., Wojnárovits L., Radiolysis of paracetamol in dilute aqueous solution, Radiation Physics and Chemistry, 2012, 81, 9, 1503-1507
H4. Homlok, R., Takács, E., Wojnárovits, L., Ionizing radiation induced reactions of 2,6-dichloroaniline in dilute aqueous solution, Radiation Physics and Chemistry, 2012, 81, 9, 1499-1502
Links
Home page of the Centre for Energy Research
References
[1] Cooper, W.J., Curry, R.D., O’Shea, K.E. (Eds) 1998, Environmental Applications of Ionizing Radiation. Wiley, New York
[2] Pikaev, A.K., in Coopoer, W.J., Curry, R.D., O’Shea, K.E. (Eds) 1998, Environmental Applications of Ionizing Radiation, Wiley, New York. 495
[3] Han, B., Ko, J., Kim, J., Kim, Y., Chung, W., Makarov, I.E., Ponomarev, A. V., Pikaev, A. K., Combined electron-beam and biological treatment of dyeing complex wastewater. Pilot plant experiments, Radiat. Phys. Chem., 2002, 64, 1, 53-59
[4] Spinks, J.W.T., Woods, R.J., 1990. An Introduction to Radiation Chemistry, third ed. Wiley-Interscience, New York
[ 5] Jovanovic, S. V., Hara, Y., Steenken, S., Simic, M. G., Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis Study, J. Am. Chem. Soc., 1995, 117, 9881-9888
[6] Farré, M., Ferrer, I., Ginebreda, A., Figueras, M., Olivella, L., Tirapu, L., Vilanova, M., Barceló, D., Determination of drugs in surface water and wastewater samples by liquid chromatography-mass spectrometry: methods and preliminary results including toxicity studies with Vibrio fischeri, J. Chromatogr. A, 2001, 938, 1-2, 187-197
[7] Sixt, S., Altschuh, J., Brüggemann, R., Quantitative structure-toxicity relationships for 80 chlorinated compounds using quantum chemical descriptors, Chemosphere, 1995, 30, 12 2397-2414
