 |
BMe Research Grant |

|
BME, Oláh György Doctoral School
Department of Organic Chemistry and Technology
Supervisors: Dr. György Keglevich and Dr. Elemér Fogassy
The synthesis of novel platinum catalysts and their application in enantioselective hydroformylation reactions
Introducing the research area
In our research, we investigated the synthesis of novel platinum catalysts that can be potentially applied in enantioselective hydroformylation reactions in the pharmaceutical industry. Methods were developed for the preparation of various enantiomers of five- and six-membered phosphorous-heterocycles - a key step to obtain the corresponding novel platinum catalysts.
Brief introduction of the research place
Organophosphorous chemistry and the separation of enantiomers are studied by two research groups in the Department of Organic Chemistry and Technology of BUTE. The two groups have been collaborating for several years in the preparation of the enantiomers of organophosphorous compounds which have potential biological activity and can serve as starting materials for catalysts. Our research group works in collaboration with other groups of BUTE, University of Pécs and Research Centre of Natural Sciences, to determine the absolute configuration of enantiomers of organophosphorous compounds and to test newly prepared catalysts.
History and context of the research
Chiral compounds form an important group in chemistry for their unique property of being non-superimposable with their mirror image. The two mirror images are called enantiomers, and they are distinguished from each other by the (R)- or (S)- descriptors. All physical and chemical properties of the enantiomers are identical, so their separation is a challenge.
Chirality is not peculiar to molecules, as there are many chiral objects in nature, for instance our left and right hands are also non-superimposable (Figure 1).
Figure 1: Chiral and achiral objects
The enantiomers of chiral compounds may have diverse effect in human body when bound to chiral receptors. For example, the (R)- or (S)-asparagine have different flavour.
Furthermore, one enantiomer of a drug may be effective, while the other one is ineffective or even toxic, as it was shown by the “Contergan scandal”. The effective agent of the Contergan drug was Thalidomide, which was introduced to the market as a 1:1 mixture of the two enantiomers (racemic form). However, while one of them was sedative, the other one was teratogenic (Figure 2). This incident transformed drug research, and it became obligatory to investigate the effect of both enantiomers separately. The above examples clearly demonstrate that enantiomers separation must have become a focal area in pharmaceutical industry as well.
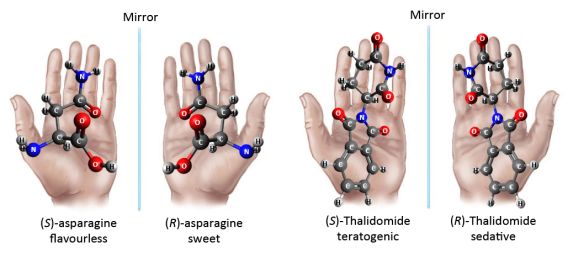
Figure 2: The difference in effect of the enantiomers of asparagine or Thalidomide
Aims of the research
Organophosphorous compounds play important role in living organisms, as well as in synthetic organic chemistry [1]. There are many organophosphorous compounds with proven biological activity, and besides, the transition metal complexes of chiral phosphines are used as catalysts in enantioselective hydrogenation and hydroformylation reactions [2-4]. An enantioselective hydroformylation step can be used in the synthesis of several non-steroidal anti-inflammatory drugs, such as Ibuprofen, Naproxen and Ketoprofen. This synthetic pathway meets many criteria of green chemistry [5].
During the research, our aim was to prepare the enantiomers of organophosphorous compounds that have potential biological activity. We also intended to synthesize potential catalysts for enantioselective hydroformylation (Figure 3).
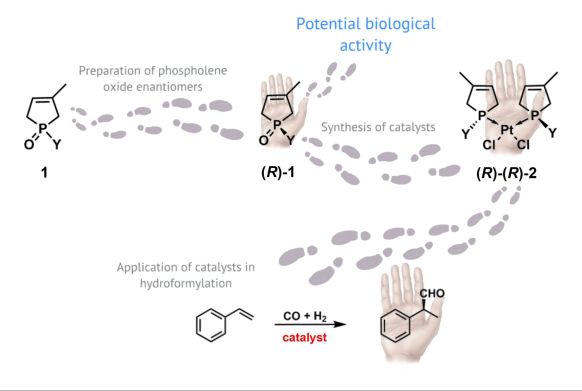
Figure 3: Aims of the present research
In our research, we focused on organophosphorous-heterocycles, and primarily on phospholene oxides (1). It has been found that the halogenated derivatives of phospholene oxides have anti-cancer effect in racemic form, however, the corresponding enantiomers have not been investigated as yet [6]. Therefore, we developed resolution methods for phospholene oxides - probably a key step to obtaining proper optically active derivatives exhibiting anti-cancer effect in enantiopure form.
The other aim of this research was to convert the enantiomers of phospholene oxides [(R)-1] to optically active platinum complexes [(R)-(R)-2] and to use them as catalysts in enantioselective hydroformylation reactions. The rhodium or platinum complexes of chiral phosphines are well established catalysts in hydroformylation. The price of rhodium was five-times the price of platinum before the financial crisis, which explains reason for the research of platinum catalysts (Figure 4).
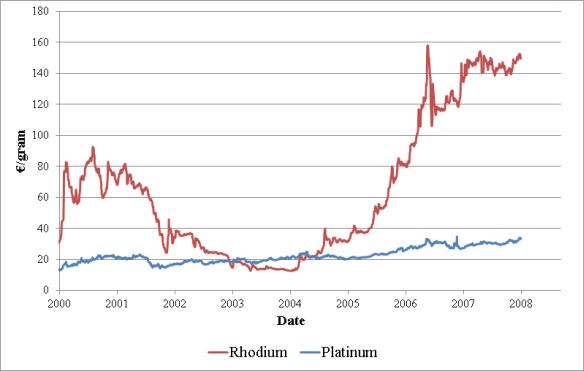
Figure 4: Development of the price of rhodium and platinum
Bidentate phosphine ligands having chiral backbone instead of a chiral phosphorous atom are frequently used in chemical industry [4, 7]. A recent review pointed out that the application of catalysts incorporating monodentate ligands may be advantageous in some reactions [8], so we included in our research the applicability of monodentate phospholene ligands with phosphorous asymmetric center in hydroformylation.
Methodology
All compounds were synthesized using modern methods of synthetic organic chemistry. The purity of compounds was checked with thin layer chromatography. The catalytic reactions were elaborated in an autoclave at a given pressure and temperature. We applied nuclear magnetic resonance spectroscopy (NMR) and high resolution mass spectrometry for the verification of chemical structure of synthesized compounds.
The enantiomeric excess of the phospholene oxide enantiomers [(R)-1] as well as product composition obtained in the catalytic hydroformylation were determined by gas or liquid chromatography using chiral stationary phases. During chromatography, the sample is driven through the stationary phase by the mobile phase. Different compounds in the sample form different interactions with the stationary phase, which can be utilized for separation. As enantiomers can be separated using a chiral stationary phase, enantiomeric excess can be determined in this manner [9].
The absolute configuration of the phospholene oxide enantiomers was determined by single crystal X-ray crystallography and CD spectroscopy. In single crystals, molecules form a periodic array, where X-ray beam diffracts and interferes on interacting with the single crystal. From the detected interferogram, the three-dimensional structure of molecules, and hence, the absolute configuration of enantiomers can be determined.
CD spectroscopy can also be used to determine the absolute configuration of enantiomers. As enantiomers absorb clockwise and counter-clockwise circular polarized light differently, a spectrum specific to the corresponding enantiomer will be detected. The absolute configuration of the enantiomer is determined by comparing the measured CD spectrum with the spectra of other derivatives of that compound or spectra obtained by quantum chemical calculations [10].
Results
Our research group has recently developed efficient resolution methods for preparing the enantiomers of phospholene oxides [(R)-1], but in the case of alkyl-phospholene oxides the enantiomeric excess values remained low [11, B2].
During my research, I prepared the enantiomers of several alkyl-phospholene oxides [(R)-1d-f] with enantiomeric excesses above 90% [B3, B6, B7]. The main challenge at the resolution of these compounds [(R)-1d-f] was that the alkyl chains could form only weak non-covalent interactions with the resolving agent, which often led to poor enantiomeric excess values. So the optimization of the reaction parameters (resolving agent and its quantity, solvent and its quantity, crystallization time) was important. The resolutions of several six-membered phosphorous-heterocycles (3-5) were also investigated, and the enantiomers of these compounds (3-5) were prepared first time in the literature (Figure 5) [B5, B10].
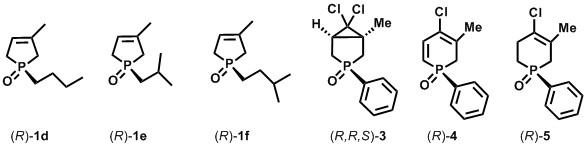
Figure 5: Enantiomers of the synthesized five- and six-membered phosphorus heterocycles
Several alkyl-phospholene oxides [(R)-1d-f] were prepared in enantiopure form, and the absolute configuration of these enantiomers was determined by CD spectroscopy and single crystal X-ray crystallography. The crystallographic data allowed us to investigate the interactions between phospholene oxide and resolving agent (Figure 6).
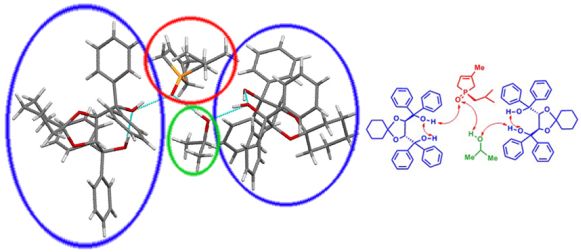
Figure 6: Interactions between isobutyl-phopholene oxide [(R)-1e] and resolving agent
The enantiomers of phospholene oxides [(R)-1] were converted to novel platinum catalysts [(R)-(R)-2] (Figure 7). Contrary to the catalysts described in literature, our novel platinum catalysts [(R)-(R)-2] incorporate monodentate ligands with phosphorous asymmetric centre. Several derivatives having alkyl groups [(R)-(R)-2b-f] were also synthesized to evaluate if these substituents have an impact on the selectivity in hydroformylation compared to the phenyl group [4].
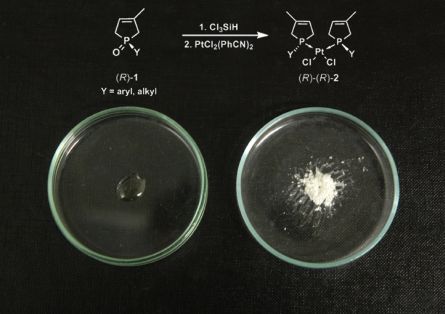
Figure 7: The synthesis of the catalysts [(R)-(R)-2]
The catalysts [(R)-(R)-2] were tested in the hydroformylation reaction of styrene (6) that was a model reaction for the synthesis of several non-steroidal anti-inflammatory drugs, such as Ibuprofen, Naproxen, Ketoprofen (Figure 8). In this reaction, the styrene reacts with carbon-monoxide and hydrogen in the presence of a catalyst to afford 2-phenylpropanal (7), its regioisomer the 3-phenylpropanal (8) and ethylbenzene (9) as a side-product. There is an asymmetric center in the 2-phenylpropanal (7), so application of chiral catalysts can lead to the formation of only one enantiomer of 2-phenylpropanal [(S)-7].
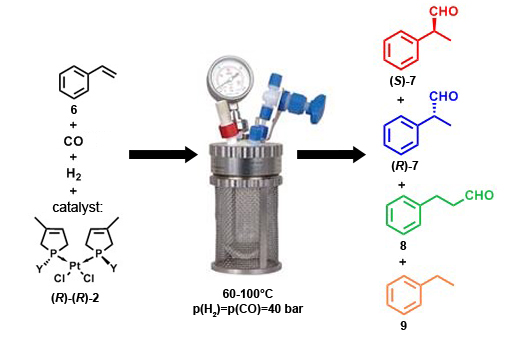
Figure 8: Hydroformylation of styrene (6)
The selectivity of catalyst has a major impact on product composition. Chemo-, regio- and enantioselectivity can be distinguished. The aim in this reaction is to synthesize one enantiomer of 2-phenylpropanal [(S)-7], which requires high chemo-, regio- and enantioselectivity.
Based on our results, it can be concluded that platinum complexes incorporating monodentate phospholene ligands [(R)-(R)-2] can be used as catalysts in the enantioselective hydroformylation of styrene (6) with high chemo- and regioselectivity (83-92% and 64-86%, respectively), but enantioselectivity values remained low (3-29%) (Figure 9). However, in comparison with the phenyl-group, alkyl groups on the phospholene moiety increased the regio- and enantioselectivity significantly (Figure 9 a and b-f). A tendency can be found between the enantioselectivity of the catalyst [(R)-(R)-2] and the length and bulkiness of the alkyl-group on the phospholene moiety [12, B3, B6].
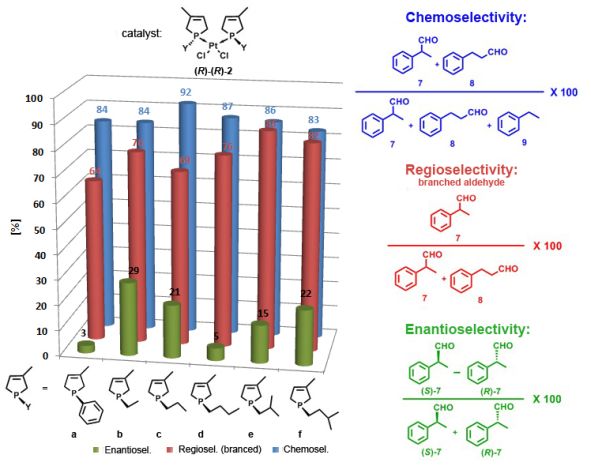
Figure 9: Catalytic results obtained using phospholene-platinum complexes [(R)-(R)-2]
Expected impact and further research
In this research, enantiomers of several five- and six-membered phosphorous-heterocycles (1d-f and 3-5) were synthesized. The results extend our knowledge on the chiral-chiral recognition of organophosphorus compounds, which can be useful in the future in the separation of other chiral organophosphorus compounds with industry application or biological activity potential. Novel phospholene platinum complexes [(R)-(R)-2] were synthesized and used as catalysts in enantioselective hydroformylation. The effect of different groups on the phospholene moiety was investigated, and we found that alkyl-substituted derivatives [(R)-(R)-2b-f] had higher regio- and enantioselectivity than that of the phenyl-derivative [(R)-(R)-2a].
Results of the research were summarized in 6 articles published in international scientific journals, and further 2 are under publication. I am also a co-author of a book chapter and a patent. I have also participated in 11 international or Hungarian conferences with oral presentations or posters.
As a next step, the synthesis and the application of other platinum catalysts incorporating six-membered heterocycles (3-5) or other alkyl-phospholenes are planned with the aim to understand the correlation between the selectivity and the structure of these catalysts.
Publications, references, links
Publications
[B1] Ujj, V.; Bagi, P.; Laki, A.; Fogassy, E.; Keglevich, G., Phosphorus. Sulfur, Silicon Relat. Elem. 2010, 186, 792.
[B2] Ujj, V.; Bagi, P.; Schindler, J.; Madarász, J.; Fogassy, E.; Keglevich, G., Chirality 2010, 22, 699.
[B3] Keglevich, G.; Bagi, P.; Szöllősy, Á.; Körtvélyesi, T.; Pongrácz, P.; Kollár, L.; Drahos, L., J. Organomet. Chem. 2011, 696, 3557.
[B4] Bagi, P.; Kovács, T.; Laki, A.; Fekete A.; Fogassy, E.; Keglevich, G., Phosphorus, Sulfur, Silicon Relat. Elem. 2013, 188, 36.
[B5] Bagi, P.; Laki, A.; Keglevich, G., Heteroatom Chem. 2013, 24, 179.
[B6] Bagi, P.; Kovács, T.; Szilvási, T.; Pongrácz, P.; Kollár, L.; Drahos, L.; Fogassy, E.; Keglevich, G., J. Organomet. Chem. 2013, in press
[B7] Bagi, P.; Fekete; A.; Kállay, M.; Hessz, D.; Kubinyi, M.; Holczbauer, T.; Czugler, M.; Fogassy, E.; Keglevich, G., Resolution of 1-n-butyl-, 1-n-propoxy-3-methyl-3-phosphole 1-oxides 2013, under publication
[B8] Bagi, P.; Szilvási, T.; Pongrácz, P.; Kollár, L.; Drahos, L.; Fogassy, E.; Keglevich, G., Platinum(II) complexes incorporating racemic and optically active 1-aryl-3-phospholene P-ligands 2013, under publication
Book chapter
[B9] Keglevich, G; Bagi, P.; Bálint, E., Platinum Compounds, Production and Applications. Varrennikov, L., Yedemsky, E., Eds. Nova Science Publishers New York, 2013, p 83.
Patent
[B10] Bagi; P.; Fogassy, E.; Keglevich, G. Hungarian Patent 1200228, 2012, submitted
11 oral presentations or posters on international or Hungarian conferences
Links
References
[1] Quin, L. D., A guide to organophosphorus chemistry. John Wiley & Sons: New York, 2000
[2] Brunner, H.; Zettlmeier, W., Handbook of enantioselective catalysis with transition metal compounds. VCH: Wenheim, 1993
[3] Botteghi, C.; Marchetti, M.; Paganelli, S., Eds., Wiley-VCH: Weinheim, 1998; Vol. 2.
[4] Kollár, L.; Keglevich, G., Chem. Rev. 2010, 110, 4257.
[5] Franke, R.; Selent, D.; Börner, A., Chem. Rev. 2012, 112, 5675.
[6] Yamada, M.; Yamashita, M.; Suyama, T.; Yamashita, J.; Asai, K.; Niimi, T.; Ozaki, N.; Fujie, M.; Maddali, K.; Nakamura, S.; Ohnishi, K., Bioorg. Med. Chem. Lett. 2010, 20, 5943.
[7] Agbossou, F.; Carpentier, J.-F.; Mortreux, A., Chem. Rev. 1995, 95, 2485.
[8] Lagasse, F.; Kagan, H. B., Chem. Pharm. Bull. 2000, 48, 315.
[9] Pokol (Ed.), G., Analitikai kémia; Typotex Kft.: Budapest, 2011
[10] Tóth, G.; Balázs, B., Szerves vegyületek szerkezetfelderítése. Műegyetemi Kiadó: Budapest, 2005
[11] Novák, T.; Ujj, V.; Schindler, J.; Czugler, M.; Kubinyi, M.; Mayer, Z. A.; Fogassy, E.; Keglevich, G., Tetrahedron: Asymmetry 2007, 18, 2965.
[12] Pongrácz, P.; Kollár, L.; Kerényi, A.; Kovács, V.; Ujj, V.; Keglevich, G., J. Organomet. Chem. 2011, 696, 2234.
