 |
BMe Research Grant |

|
BME, Oláh György Doctoral School
Department of Inorganic and Analytical Chemistry
Supervisor: Dr. László Nyulászi
Ionic liquids and carbenes - New possibilities?
Introducing the research area
The chemistry of ionic liquids [1] and carbenes [2] is intensely studied (there are ~5,000 citations for ionic liquids and ~2,000 for carbenes according to Web of Science). During my Ph.D work, I investigated the connection of these two “hot” areas, which could lead to considerable technological advances in this field of chemistry. One of the most characteristic properties of ionic compounds is the high melting point (sodium chloride 801 °C), however, ionic liquids (IL) (Fig. 1) with a melting point below 100 °C do exist, too. Ionic liquids have many applications. They can be used as green solvents due to their extremely low vapor pressures, and as electrically conducting fluids in solar cells or in separation techniques, too. [3]
Carbenes are of great current interest, due to their unusual structural properties (Fig. 2) and their catalytic activation in transition-metal complexes or as metal-free catalysts. Their significance as transition-metal complexes [4] is clearly demonstrated by two Nobel prizes awarded in this area (Fisher - 1973; Schrock, Chauvin, Grubbs - 2005).
During the last few years, free carbenes were widely used as organocatalyst, and this important field was under a rapid and continuous development.
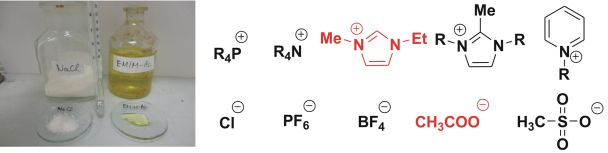
Fig.1: Two ionic compounds: NaCl (solid) EMIM-Ac (liquid) and commonly used cations and anions for ionic liquids
Brief introduction of the research place
My doctoral studies were carried out in the group of Prof. László Nyulászi at the BUTE, Faculty of Chemical Technology and Biotechnology, Department of Inorganic and Analytical Chemistry. This computational chemistry group has a long standing experience in the fields of main group and carbene chemistry as well as in ionic liquid research. Our international collaborations and several publications in leading journals indicate the high level of our research. Synthetic work has been carried out in the experienced group of Dr. József Nagy (Department of Organic Chemistry and Technology).
History and context of the research
The importance of environmentally benign industrial processes is continuously increasing. During chemical processes, some organic solvents may unavoidably evaporate into the atmosphere causing environmental concerns. Use of ionic liquids, which are composed of organic cation and organic or inorganic anion (Fig. 1) is a possible way to reduce the emission of organic solvents, furthermore, their properties can be varied by simple changes in the structure of ions. Although in most of these reactions, IL is merely the solvent, there are a few reports showing that imidazolium-based ionic liquids can be used as precatalysts for N-heterocyclic carbene catalyzed reactions (Fig. 2), whereby the catalyst can be obtained from the imidazolium cation by deprotonation with an external base.
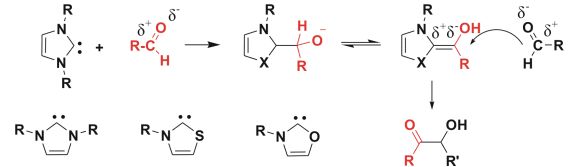
Fig.2: N-heterocyclic carbenes and the mechanism of the carbene-catalyzed benzoin condensation
Catalysts that increase the rate of a chemical reaction are also preferred in environmentally friendly green chemistry [5]. Their most important role is providing an alternative reaction pathway which has a lower activation energy to the reaction product. Organocatalysts are of specific importance, because there is no need for expensive, rare and toxic transition metals as the catalyst is an organic molecule. Carbenes are among the organocatalysts and are known to catalyze highly important reactions, e.g. the biometic benzoin condensation imitating a biochemical reaction catalyzed by vitamin B1 within the transketolase enzyme. Due to their unique electronic properties [6], carbenes reverse the polarity of a chemical bond (umpolung) modifying its reactivity (Fig. 2), which is a powerful strategy in organic synthesis.
Over the past years, several carbene-catalyzed redoxreaction have been published in the literature. [7] Highly interesting reactions are those, where carbon-dioxid is used as an oxidant. Besides the chemical curiosity of this reaction, this process is also important in view of the environmental significance of re-using carbon-dioxide.
The research goal, open questions
It was shown in earlier studies that the connection between the ionic liquids and carbenes was made by the added base. Our group had previously detected the presence of carbene in gas phases photoelectron and mass spectroscopies from EMiM-Ac [8], however, the amount of carbene in the liquid phase still may not be enough to provide the organocatalytic activity or possibly carbon-dioxide reduction. While the observed [9] excellent solubility of CO2 in EMIM-Ac is apparently favourable for the reactivity, the reasons for the solubility and the mechanism of carbon-dioxide reduction are far from being understood. [4 (e), (f), (g) ]
It is apparent that the variation of cationic precursors can have beneficially influence on the properties such as the catalytic activity or CO2 solubility. Thus, we investigated computationally other synthesized carbenes and also unknown systems. The question arises in the case of transketolase enzyme, that why nature specifically “chose” thiazole-2-ylidene, and not imidazol-2-ylidene, e.g.[10] (a more stable compound, and the first reported NHC) or the oxygen analogue oxazol-2-ylidene? Interestingly, this latter carbene has not been synthesized yet, although several other carbenes containing nitrogen and oxygen are known from literature. [11] To answer this problem, possible chemical decomposition reactions such as hydrolysis should also be considered.
Methodology
During my work I combined the state-of-the-art methods of theoretical and preparative organic chemistry. Quantum chemical calculations were carried out using high performance computers. I use the newest DFT methods for modelling. Before using a quantum chemical model, the eligibility of the model needs to be checked. Therefore, preliminary calculations were made to choose the proper model which describes best our system. The appropriateness of a model can best be proven by the successful follow up experiments. Accordingly, I do experiments in the group of Dr. József Nagy. The structure and purities of synthesized compounds were determined by thin layer chromatography (TLC), gaschromatogrphy mass spectrometry GC-MS, nuclear magnetic resonance (NMR) and infrared spectroscopy (IR). Further experiments are carried out in high pressure reactor collaborating with Dr. Edit Székely.
Results
1-Ethyl-3-methylimidazolium acetate (EMIM-Ac) ionic liquid as versatile organocatalytic ionic liquid [K1]
During my work, I investigated the formation of carbenes in the liquid phase of EMIM-Ac ionic liquid. (1. Fig) In our previous study we had shown that with basic anions (e.g. acetate) imidazolium salts can be deprotonated in the gaseous phase resulting in carbenes.[2] My preliminary calculations showed that the energy of neutral carbenes containing structures increased due to the interaction with the surrounding charged particles stabilizing the ionic form. (Fig. 3)

Fig.3: The interaction with the surrounding charged particles stabilizes the ionic form.
There is no spectroscopy evidence for the formation of carbene, nevertheless, the carbene could be trapped [12]. During my preparative work, I showed clearly the organocatalytic activity of EMIM-Ac in several reactions. Importantly, carbon monoxide, the byproduct of the disputed carbene catalyzed carbon-dioxide reduction was successfully detected several times by IR-spectroscopy, although it should be noted that we had some problems with the reproducibility of this reaction. Understanding these reactions is still an ongoing part of our research.
To understand the mechanism of carbon-dioxide reduction, first I investigated the behaviour of carbon-dioxide in EMIM-Ac. Carbon-dioxide is soluble both physically and chemically. According to the generally accepted view is that while the anion plays a crucial role in the solute–solvent interplay [13], the cation–carbon-dioxide interaction is merely limited to small contributions from the side chain, and so far no significant direct effect of the cationic head group has been reported. Collaboration with the group of Professor Barbara Kirchner has revealed non-negligible interactions between carbon-dioxide and imidazolium cation. We can thus modify the general picture to consider that only anionic interaction plays important role in the solubility of carbon-dioxide. [K2]
During the investigation of the chemical solubility of carbon-dioxide, my calculations showed that the 4- and 5-carboxylate (Fig. 4 B and C) are thermodynamically more stable than 2-carboxylate (A). In collaboration with Dr. Edit Székely, a high pressure experiment was carried out which showed that not only 2-carboxylate but 4- and 5-carboxylate were also formed.
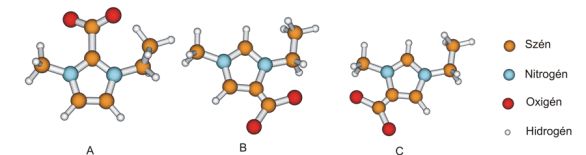
Fig.4: Possible product of the reaction of the carbon-dioxide and imidazolium cation
Role of Sulphur in Thiamine [K4]
First, we focused on the proton affinities of the different carbenes related to the first step of most NHC organocatalytic processes, namely the formation of the catalyst from its salt (which is the IL). The protonation energies exhibited significant substituent effects according to the atoms attached to the hypovalent carbon. In the case of the synthetically most important five-membered rings, the obtained high proton affinity decreases in the order of N > S > O. Furthermore, the Breslow intermediate has been found to be by 10 kcal/mol more stable for thiazol-2-ylidene than for imidazol-2-ylidene (Fig 2). Moreover, thiazol-2-ylidene ring can open reversibly with an excess of water that may provide better transport through cell membranes. These results can help understand why nature “chose” thiazole-2-ylidene in the transketolaze reaction.
Stability of oxazol-2-ylidene.[K5]
I showed that oxazol-2-ylidene exhibits the second largest stabilization energy exceeding the stabilization of some already synthesized carbenes such as thiazol-2-ylidene.
To assess the overall stability of carbene, however, not only thermodynamical aspects, but also possible chemical decomposition reactions were considered. Thus, the fragmentation of oxazol-2-ylidene (Fig. 5) to acetylene and an isocyanate by a cycloreversion were studied, and our results showed that this compound can be stabilized by substitutions.
These compounds were calculated to be stable in dimerization and in cycloreversion reactions, provided that the water content of the reaction mixture - dissolved in the ionic liquid- was significantly reduced. As a new carbene, it can open a new perspectives in the fine-tuning of catalytic reactions.
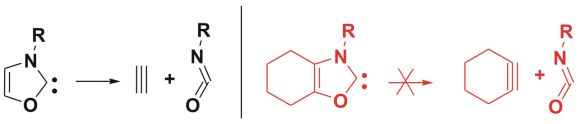
Fig.5: Cycloreversion reactions of oxazol-2-ylidene and a suitable target for synthesis, which should be stable in dimerization and in cycloreversion reactions
Expected impact and further research
During my work, I pointed out that the carbene concentration in 1-ethyl-3-methylimidazoliumacetate ionic liquid was sufficiently high to act as organocatalyst. This observation reveals the potential of ionic liquid organocatalysts, uniting the beneficial properties of these two families of compounds.
We have published these results in the Organic and Biomolecular Chemistry [K1], and over two months, this article was among the most frequently visited ten articles, and there have been 12 citations until now. It should be noted that one of these articles suggests a biomass utilization based on the organocatalytic activity of these ionic liquid. [14]
More results have been published in leading international journals. [K2, K4, K5, K6] Furthermore, one article will be published in collaboration with Prof. Rainer Streubel [K7), and one will be published in collaboration with Dr. Edit Székely [K3].
Publications, references, links
Publications
[K1] Z. Kelemen, O. Hollóczki, J. Nagy, L. Nyulászi, Org. Biomol. Chem., 2011, 9, pp. 5362-5364.
[K2] O. Hollóczki, Z. Kelemen, L. Könczöl, D. Szieberth, L. Nyulászi, A. Stark, B. Kirchner, ChemPhysChem 2013, 14, pp. 315-320
[K3] Z. Kelemen, B. Péter-Szabó, E. Székely, J. Nagy, L Nyulászi, to be published
[K4] O. Hollóczki, Z. Kelemen, L. Nyulászi, J. Org. Chem., 2012, 77, pp. 6014–6022
[K5] Z. Kelemen, O. Hollóczki, J. Oláh, L. Nyulászi, RSC Advances, 2013, 3, pp. 7970-7978
[K6] P. K. Majhi, G. Schnakenburg, Z. Kelemen, L. Nyulászi, D. Gates, R. Streubel Angew. Chem. Int. Ed. DOI: 10.1002/anie.201304431
[K7] Z. Kelemen, R. Streubel, L. Nyulászi to be published
Links
References
[1] (a) J. P. Hallett, T. Welton, Chem. Rev, 2011, 111, 3508-3576 (c) T. Welton, Chem. Rev, 1999, 99, 2071-2083. (c) P. Wasserscheid, T. Welton Ionic Liquids in Synthesis 2. Edition 2007, Wiley-VHC Verlag, Weinheim (d) Z. Finta, Z. Baán, I. Hermecz, Kémiai Újabb Eredményei 2007 98, Akadémiai kiadó, Budapest. (e) J. P. Hallett, T.Welton, Chem. Rev., 2011, 111, 3508-3576
[2] (a) D. R. MacFarlane, M. Forsyth, P. C. Howlett, J. M. Pringle, J. Sun, G. Annat, W. Neil, E. I. Izgorodina, Acc. Chem. Res. 2007, 40, 1165 (b) E. F. Borra, O.Seddiki, R. R. Angel, D. Eisenstein, P. Hickson, K. R. Seddon, S. P. Worden, Nature 2007, 447, 979. (c) X. Han, D. W. Armstrong, Acc. Chem. Res. 2007, 40, 1079. (d) R. P. Swatloski, S. K. Spear, J. D. Holbrey, R. D. Rogers, J. Am. Chem. Soc. 2002, 124, 4974
[3] (a) D. R. Anderson, V. Lavallo, D. J. O’Leary, G. Bertrand, R. H. Grubbs, Angew. Chem. Int. Ed. 2007, 46, 7262. (b) R. H. Crabtree, J. Organomet. Chem. 2005, 690. 5451. (c) S. Diez-González, N. Marion, S. P. Nolan, Chem. Rev. 2009, 109, 3612. (d) K. H. Dötz (Ed) Metal Carbenes in Organic Synthesis 2004, 13 Springer, (e) R. H. Grubbs, Handbook of Metathesis 2003 Wiley-VCH, Germany
[4] (a) D. Bourissou, O. Guerret, F. P. Gabbai, G. Bertrand, Chem. Rev. 2000, 100, 39-92. (b) F. E. Hahn, M. C. Jahnke, Angew. Chem. Int. Ed. 2008, 47, 3122-3172 (c) A. J. Arduengo III, G. Bertrand, Chem. Rev. 2009, 109, 3209. (d) J. Vignolle, X. Cattoen, D. Bourissou, Chem. Rev. 2009, 109, 3333
[5] T. P. Anastas, J. C. Warner: Green Chemistry: Theory and Practise, Oxford University Press, Oxford, 1988
[6] D. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, pp. 389-399
[7] (a) J. H. Park, S. V. Bhilare, S.W. Youn, Org. Lett., 2011, 13, 2228; (b) J. H. Park, S. V. Bhilare S.W. Youn, Org. Lett., 2011, 13, 2228 (d) S. N. Riduan, Y. Zhang, J. Y. Ying, Angew. Chem., Int. Ed., 2009, 48, 3322. (e) P.-C. Chiang, J.W. Bode, Org. Lett., 2011, 13, 2422 - 2425 (f) M. Yoshida, Y. Katagiri, W.-B. Zhu, K. Shishido Org. Biomol. Chem., 2009, 7 , 4062 – 4066 (g) B. Maji, S. Vedachalan, X. Ge, S. Cai, X. Liu J. Org. Chem. 2011, 76, 3016–3023 (h) V. Nair, V. Varghese, R. R. Paul, A. Jose, C. R. Sinu, R. S. Menon, Org. Lett., 2010, 12, 2653. (i) L. Gu, Y. Zhang, J. Am. Chem. Soc., 2010, 132, 914
[8] O. Hollóczki, D. Gerhard, K. Massonne, L. Szarvas, B. Németh, T. Veszprémi, L. Nyulászi, New. J. Chem. 2010, 34, 3004-09
[9] L. A. Blanchard, D. Hancu, E. J. Beckman, J. F. Brennecke, Nature 1999, 399, pp. 28–29
[10] A. J. Arduengo III., R. L. Harlow, M. Kline, J. Am. Chem. Soc. 1991, 113, 361
[11] (a) R. W. Alder, C. P. Butts and A. G. Orpen, J. Am. Chem. Soc., 1998, 120, pp. 11526–11527; (b) N. Merceron Saffon, A. Baceiredo, H. Gornitzka and G. Bertrand, Science, 2003, 301, 1223
[12] (a) H. Rodríguez, G. Gurau, J. D. Holbrey, R. D. Rogers, Chem. Commun., 2011, 47, 3222. (b) M. Yoshizawa-Fujita, K. Johansson, P. Newmann, D. R. MacFarlane, M. Forsyth, Tetrahedron Lett., 2006, 47, 2755. (c) C.-M. Jin, B. Twamley, J.M. Shreeve, Organometallics, 2005, 24, 3020
[13] (a) B. L. Bhargava, S. Balasubramanian, Chem. Phys. Lett. 2007, 444, pp. 242–246. (b)C. Cadena, J. L. Anthony, J. K. Shah, T. I. Morrow, J. F. Brennecke, E. J. Maginn, J. Am. Chem. Soc. 2004, 126, pp. 5300 –5308
[14] D. Liu, Y. Zhanga E.Y.-X. Chen Green Chem., 2012, 14, 2738
