 |
BMe Research Grant |

|
Oláh György Doctoral School
Department of Organic Chemistry and Technology
Supervisor: Dr. György Keglevich
Microwave-promoted environmentally friendly synthesis
Introducing the research area
Nowadays, green chemistry is becoming of increasing importance. According to the twelve principles of green chemistry, synthetic methods should be designed to use and generate substances with little or no toxicity to human health and the environment, while minimizing the incorporation of all materials used in the process into the final product. Atom and energy efficiency, as well as sustainability are also important aspects. Unnecessary derivatizations should be minimized or avoided if possible, thus the shortest reaction path should be chosen. In the context of green chemistry, microwave (MW)-assisted organic synthesis is a challenging field. Every little contribution to these principles can make a difference.
Brief introduction of the research place
Our research group operating at the Department of Organic Chemistry and Technology is led by prof. György Keglevich, and engaged with environmental friendly and organophosphorus chemistry, has a history of more than fifteen years, performing important industrial streamlining reactions and producing new, potentially bioactive organophosphorus compounds of fine chemical and pharmaceutical importance. Our numerous publications indicate high level of research.
History and context of the research
Microwave (MW) technology has gone through remarkable development over the last 2-3 decades, while becoming more and more popular among chemists. The first papers on MW-assisted organic chemistry were published in 1986 [1,2]. Early research was performed in modified domestic MW ovens which proved problematic: lack of safety controls and temperature monitoring resulted in hardly reproducible reactions. Moreover, domestic cavities could not withstand the explosive force in runaway reactions. Since the early 2000’s MW reactors dedicated specifically to laboratory usage have become available, equipped with temperature and pressure monitoring and safety controls. This has resulted in a boost of research, demonstrated by an increased number of publications within year-over-year growth rates (Fig. 1) [3].
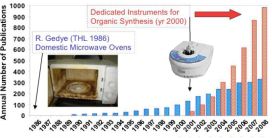
Fig. 1: Increased number of publications since the availability of dedicated MW reactors
Among the numerous benefits of MW technology application (see below) is its significant contribution to the implementation of green chemistry principles, through avoiding the usage of solvents, reducing the energy usage, and making chemical modifications more efficient.
The research goal, open questions
During my work, our goal was to study transformations which practically cannot be enforced by conventional heating, yet can be accomplished by MW irradiation. In such cases, MW technique enables the elicitation of harmful reagents by implementing solventless and catalyst-free methods.
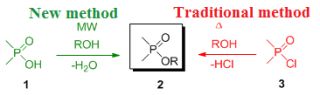
One example of the investigated reactions is the direct esterification of phosphinic acids (1) with alcohols, which do not take place under traditional conditions. Hence, the preparation of the desired esters – called phosphinates (2) – involves the use of phosphinic halogenides (3) that cannot be regarded as “green” and requires expensive P-reagents.
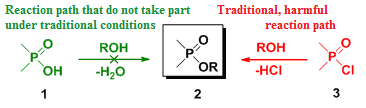
Despite it's drawbacks in terms of environmental friendliness and costs, it is widely applied in industry, since organophosphorus compounds find applications as agrochemicals and medicines.
It was a challenge for us to investigate the possibility of the above mentioned reactions under MW-assisted conditions.
Another objective was to understand and interpret how microwaves promote organic syntheses, as so-called “magic” effects are frequently attributed to MW chemistry, and existing interpretations are highly debated [4].
For a better understanding – collaborating with quantum chemists – we aimed at comparing our experimental results with theoretical calculations.
Methodology
During my daily work, the experiments were carried out in a CEM MW reactor shown in Figure 2.

Fig. 2: CEM MW reactor
Microwave (MW) itself is a form of electromagnetic energy, that falls in the 300 MHz to 30 GHz frequency range (between infrared and radio waves) with wavelengths ranging from one meter to as short as one centimeter.
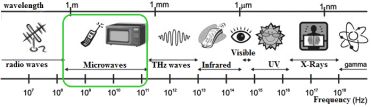
Fig. 3: Microwaves within the electromagnetic spectra
Traditionally, syntheses have been achieved through conductive heating which is a slow and inefficient process, where heat is driven into the reaction mixture, passing first through the walls of the vessel. On the other hand, microwaves couple directly with the molecules resulting in an instantaneous localized so-called “superheating” [5].
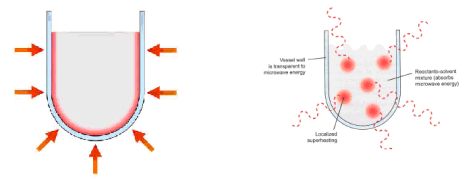
Fig. 4: Traditional (conductive) heating (![]() ) Heating by microwaves (MW)
) Heating by microwaves (MW)
In the case of MW irradiation, polar molecules try to align themselves with the changing electric field of MW – this interaction is called dipole rotation – leading to a rapid rise in temperature. Fig. 5 shows temperature gradients (after 1 min) in MW (left) versus oil-bath (right) heating.
The consequence of MW irradiation is the statistically occurring local overheating effect which can reach +30-60 °C compared to that of the bulk temperature.
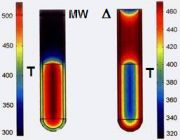
Fig. 5: Temperature gradients in MW (left) versus oil-bath (right) heating
Based on the Arrhenius reaction rate equation, one can calculate that while a typical first order reaction requires 68 days at 27 °C to reach a conversion of 90%, the same results can be achieved after 1.6 seconds carrying out the reaction at 227 °C [6]. This means that transformations being otherwise impossible can be realized under MW conditions.
A great example found by us is the direct esterification of phosphinic acids (1) which – in contrast to the analogous carboxylic acid – did not take place under traditional thermal conditions. Interestingly, the direct esterification of carboxylic acids with alcohols is the most common and widely used method to provide the corresponding esters. To understand the difference between the reactivity of carboxylic- and phosphinic acids in the course of reaction with simple alcohols, high level quantum chemical calculations have been carried out. Our studies aimed at clarifying how microwaves can drive reactions by comparing our results obtained by syntheses and the energetics calculated theoretically.
Results
We have found that phosphinic acids (1) and the excess of alcohol (also used as solvent) exposed to MW-irradiation can be effectively converted into the corresponding phosphinates (2). Based on this observation, we have developed a new, economic and environmentally friendly method for the phosphinic acid → phosphinate (1→2) transformation [K1,2].
To get an insight into the difference between the esterification of carboxylic and that of phosphinic acids, high level quantum chemical calculations have been carried out. Fig. 6 shows that while the esterification of carboxylic acids is slightly favoured, in the case of phosphinic acids there is no driving force for the reactions under discussion. Neither the thermodynamic, nor the kinetic data obtained by high level quantum chemical calculations justify the direct esterification of phosphinic acids under thermal conditions.
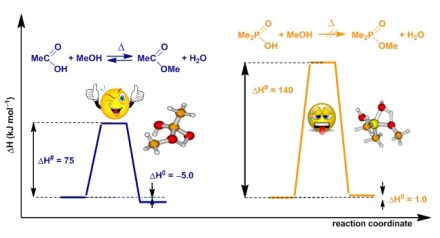
Fig. 6: Typical energy diagram for the direct esterification of carboxylic and phosphinic acids
We assumed that local overheating effect that occurs statistically can overcome the barrier corresponding to the high values of the enthalpy of activation (△H#). To justify this, comparative thermal reactions have been carried out in a bomb tube, performed in a similar way as the MW experiments (same scale, temperature, pressure and stirring). Diagram 1 shows the significant difference between the thermal and MW experiments and highlights the potential of MW technique in the synthesis of phosphinates.
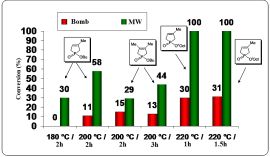
Diagram 1: Comparison of traditional and MW irradiation
Encouraged by these results, we attempted the MW-assisted direct amidation of certain phosphinic acids (1). However, these reactions could only be performed at lower conversion rates (ca. 30%). To understand this phenomena, the energetics and mechanism of direct esterifications were evaluated by high level (B3LYP/6-31++G(d,p)) calculations. Our results show that while the esterifications of the phosphinic acids are controlled kinetically, the amidations are governed by thermodynamic factors [K9].
Comparison of the results of syntheses (practice) and the energetics calculated (theory) for the esterification and amidation enabled us to draw more general conclusions on the scope and limitations of the use of MW irradiation in organic syntheses [K10].
Based on these results, we can conclude that there is no ”magic” involved in the course of MW-assisted chemistry. Simply the extra energy gained from the friction of molecules may overcome relatively high enthalpies of activation.

Fig. 7: Microwave chemistry: Myth or Reality? [4]
Expected impact and further research
Additionally to their potential biological activity, the target organophosphorus compounds possess general values in the synthesis of new P-heterocycles.
Inspired by the above-mentioned results, further transformations being impossible under conventional conditions are aimed to be studied. We have shown the potential of MW technique, however, certain limitations also apply. Scaling-up is one of the most urgent problems yet to address. Developing continuous-flow methods seems to be a plausible solution, but then the reaction mixture may not be too dense and inhomogeneous. The adaptation of our new method to continuous-flow reactor appears to be feasible. We are in the fortunate position that such equipment is already available at our Department (schematic illustration is shown in Fig. 8), making it possible for us to examine and model the scaling-up.
Fig. 8: Continuous-flow MW reactor
Publications, references, links
Publications:
[K1] N. Zs. Kiss, K. Ludányi, L. Drahos, G. Keglevich, Synthetic Commun 2009, 39, pp. 2392-2404.
[K2] G. Keglevich, E. Bálint, N. Zs. Kiss, E. Jablonkai, L. Hegedűs, A. Grün, I. Greiner, Curr Org Chem 2011, 15, pp. 1802-1810.
[K3] G. Keglevich, N. Zs. Kiss, E. Bálint, E. Jablonkai, A. Grün, M. Milen, D. Frigyes, I. Greiner, Phosphorus, Sulfur, 2011, 186, pp. 802-803.
[K4] G. Keglevich, A. Grün, E. Bálint, N. Zs. Kiss, R. Kovács, I. Greiner, I. Molnár, Zs. Blastik, V. R. Tóth, A. Fehérvári, I. Csontos, Phosphorus, Sulfur, 2011, 186, pp. 613-620.
[K5] G. Keglevich, N. Zs. Kiss, G.K. Menyhárd, A. Fehérvári, I. Csontos, Heteroatom Chem 2012, 23, pp. 171-178.
[K6] N. Zs. Kiss, A. Kaszás, L. Drahos, Z. Mucsi, G. Keglevich,Tetrahedron Lett. 2012, 53, pp. 207–209.
[K7] G. Keglevich, N. Zs. Kiss, Z. Mucsi, T. Körtvélyesi, Org. Biomol. Chem. 2012, 10, pp. 2011-2018.
[K8] G. Keglevich, A. Grün, E. Bálint, N. Zs. Kiss, E. Jablonkai, Curr. Org. Chem. 2013, 17, pp. 545-554.
[K9] G. Keglevich, N. Zs. Kiss, T. Körtvélyesi, Heteroatom Chem. 2013, in press
[K10] G. Keglevich, N. Zs. Kiss, L. Drahos, T. Körtvélyesi,Tetrahedron Lett. 2013, 54, pp. 466-469.
[K11] N. Zs. Kiss, A. Simon, L. Drahos, K. Huben, S. Jankowski, G. Keglevich, Synthesis, 2013, 45, pp. 199-204.
[K12] N. Zs. Kiss. É. V. Böttger, L. Drahos, G. Keglevich, Heteroatom Chem, 2013, 24, 283-288. page
[K13] G. Keglevich, N. Zs. Kiss, T. Körtvélyesi, Z. Mucsi, Phosphorus, Sulfur 2013, 188, 29-32 page
Links:
References:
[1] R. J. Giuerre, T. L. Bray, S. M. Duncan, Tetrahedron Lett. 1986, 27, 4945.
[2] R. Gedye, F. Smith, K. Westway, H. Ali, J. Rousell, Tetraherdron Lett. 1986, 27, 279.
[3] P. Lidstörm, J. Tinerney, B. Wathey, J. Westman, Tetrahedron 2001, 57, 9225.
[4] C. O. Kappe, B. Pieber, D. Dallinger, Angew. Chem. 2013, 52, 1088.
[5] L. B. Hayes, Microwave Synthesis Chemistry at the Speed of Light, CEM Publishing, USA, 2002, 11-25, 97-145.
[6] C. O. Kappe, Angew. Chem. Int. Ed., 2004, 43, 6250-628.
[7] D. R. Baghurst, D. M. P. Mingos, Chem. Soc. Rev., 1991, 20, 1-47.
