|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Prof. György Marosi
Solid Formulation of Biodrugs by Electrospinning
Introducing the research area
The growing importance of APIs (active pharmaceutical ingredients) of biotechnological origin means huge challenge for the pharmaceutical technologists. These biodrugs are highly sensitive to environmental factors thus traditional drying technologies (lyophilization spray drying) enable their solid formulation only at the expense of significant biological activity loss and high costs, due to their remarkable time and energy demand. In this research, we investigate whether electrospinning technology, widespread in plastic and textile industry but not yet applied in pharmaceutical industry, is suitable for replacing classic drying techniques used in the solid formulation of biodrugs.
Brief introduction of the research place
The Research Group of Safety, Environmental and Pharmaceutical Technologies (SAFEPHARMATECH) is committed to technology and materials science related researches that aim to improve the quality of life in a non-sector specific way. This involves the improvement of safe, controlled, continuous and integrated technologies applied in pharmaceutical industry, the research of fire safety and the use of renewable energy sources in automotive industry and the complex recycling of plastic waste in the field of environmental protection.
History and context of the research
The second half of the 20th century has seen huge advances in the field of biotechnology and the tendency has continued unabated in the 21st century broadening the pharmaceutical palette with various APIs (active pharmaceutical ingredients) of biotechnological origin (Figure 1.). The broad spectrum of biotechnology based API-s or biodrugs includes proteins, peptides, whole cells, nucleic acids, viral particles and vaccines [1] all being produced in a biotechnological way where the end product, the biodrug itself is usually in a water solution or suspension.
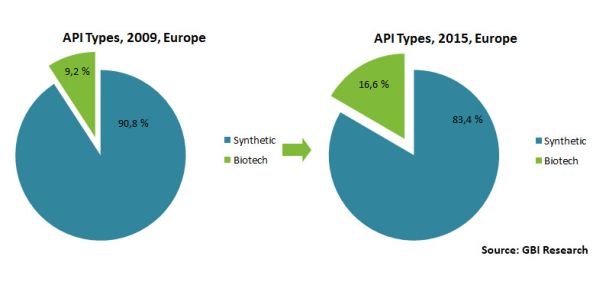
Figure 1. Growing role of APIs in Europe
Typical features of biodrugs are high sensitivity to their physical and chemical environment (temperature, pressure, mechanical stress, pH, ion concentration, etc.) and low stability in aqueous medium [2]. The gentle removal of water from these systems increases significantly the stability of the biodrugs thus providing improved transportability and shelf life.
The most usual way of water removal is lyophilization [3] which may decrease the biological activity through the irreversible damaging of the structure of the biodrug by water crystals [4]. Another disadvantage of lyophilization is that it is time-consuming and has high energy demand [5, 6]. A less widespread alternative is spray drying, where drying is provided by heated air, thus also decreasing biological activity of biodrugs usually having low thermal stability (Figure 2.).
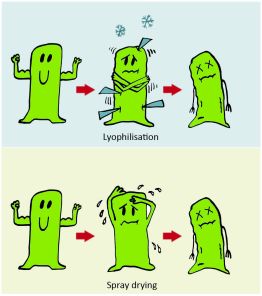
Figure 2. Conditions of freeze- and spray drying are so harmful to biodrugs
The growing expansion of biodrugs and their solid drug forms in pharmaceutical development means new challenges for pharmaceutical technologies and among all these the greatest demand for technological improvement is on the field of drying.
The research goal, open questions
In our researches, two widely used biological APIs were chosen for formulation experiments: the first one is Lactobacillus acidophilus, a probiotic bacterium, an important member of human microflora. Besides its numerous therapeutic application, nowadays it is successfully used in the treatment of the most common gynecological illness, the bacterial vaginosis (BV) [7]. The other biodrug was beta-galactosidase, an enzyme produced by the small intestine that catalyzes the hydrolysis of lactose into glucose and galactose. The absence of adequate quantity of this enzyme causes the symptoms of lactose intolerance (nausea, colic, distention and diarrhea) in which the 75% of the adult population is involved [8]. Not only have both biodrugs high therapeutic importance, but they serve as good models for the formulation experiments of the most widely used biodrugs, the probiotics and the protein APIs, medicines of diseases untreatable so far.
The aim of our research is to develop an alternative drying method instead of lyophilization that is fast, productive, continuous, has a low energy demand and is gentle enough to enable the production of solid biodrug formula.
In the case of both biodrugs, we have investigated the applicability of electrospinning, a new technology in pharmaceutical researches to produce solid drug formula from aqueous solution with the lowest loss of activity and the highest stability of the biodrug.
Methods
Lactobacillus acidophilus was fermented in MRS Broth at 37°C for two days and centrifuged. To the suspension thus obtained typical excipients of the bacterium drying were added such as trehalose, saccharose and skim milk powder. Beta-galactosidase was kindly provided by Optiferm and the concentration of the initial solution was 0.25 g/ml. The aqueous suspension or solution of the biodrug was added to the polymer solutions of which the electro spinning was carried out.
Electrospinning is a simple and effective technology to produce very fine polymer fibers with a diameter of 50-1000 nm. PVA (poly-vinyl-alcohol) and PVP (poly-vinyl-pyrrolidone) were used as polymer matrices both accepted by drug administration authorities. The optimal concentration of polymer solutions were optimized as electrospinning is only possible above a certain concentration. At low concentrations, polymer pearls appear on the collector (electro spraying), while higher concentration of the solution results in necklace-like fibers like and as we are approaching the optimal concentration, fibers become disjoint and uniform in their appearance. (Figure 3.)
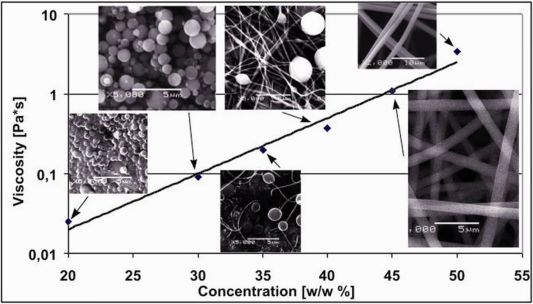
Figure 3. Morphology of nanofibers as the function of polymer concentration (PVP)
The solutions containing the polymer and the biodrug are charged to a high voltage (35 kV) with one electrode connected to a spinneret and the collector electrode grounded. Due to the high electric field, the liquid gains a conical shape called Taylor-cone from which a thin jet will emerge towards the grounded collector, as long as the Coulomb repulsion of the charges on the cone surface exceeds the retaining force of the surface tension. The emerging liquid jet is drawn by electrostatic forces, gets thinner, increases in surface, accelerating the evaporation of the solvent. The fiber-shaped solid polymer can then be collected and removed from the collector. The drying process, the production of the dry fibers from aqueous solution takes less than a second [9] (Figure 4.). Dry samples were stored at 7°C in sterile closed vessels.
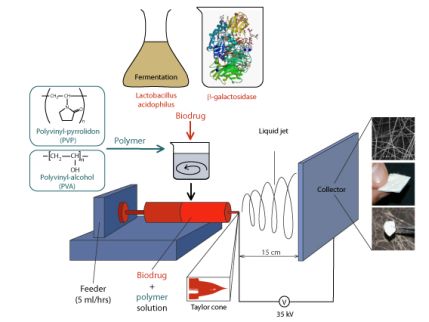
Figure 4. Solid Formulation of Biodrugs by Electrospinning
The morphology of fibers was investigated with scanning electron microscopy (SEM). To gain accurate results about the indulgence of the technology, enzyme activity and the number of colony forming units were determined in the initial aqueous solutions and in the resulting polymer fibers.
Bacterial suspensions and solid products were added gravimetrically and diluted with sterile water and dilution series were prepared. The diluted suspensions were added to MRS agar plates and incubated for 48 hours at 37°C in under anaerobe circumstances in the presence of oxygen binding material (Anaerocult) and the number of colony forming units was counted.
The activity of beta-galactosidase was investigated by the tracking of catalyzed reactions carried out at 55°C, pH 4.6 for 10 minutes with continuous stirring and stopped with 1 M sodium-carbonate solution. Two substrates were tested: orto-nitrophenyl-beta-galactoside and lactose. The former is hydrolised by the enzyme into galactose and orto-nitrophenol that gives a yellow color in alkali solution, thus it can be determined with UV-VIS spectrophotometry. The latter is natural substrate of the enzyme where the products – glucose and galactose – were quantified with HPLC (high performance liquid chromatography).
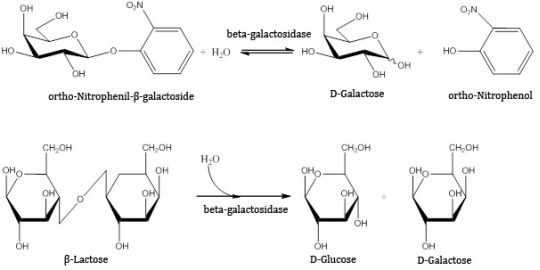
Figure 5. Reactions applied to measure the activity of beta-galactosidase
Results
In our researches we have successfully produced polymer fibers containing biodrugs. Using either fiber forming polymers, the polymer was wrapping the bacteria as a coating while the enzyme containing polymers show smaller knobs (Figure 6.). Bacteria stuck together could be observed in several cases, suggesting that the forces acting during the fiber production were insufficient for the separation of bacteria stuck together during cell division. This implies that the bacteria are merely exposed to a modest mechanical stress during the drying process.

Figure 6. Biodrugs embedded into polymer fibers (A: Lactobacillus acidophilus in PVA, B: Lactobacillus acidophilus in PVP, C: beta-galactosidase in PVP)
Our SEM results showed that the polymer fibers containing biodrugs did not have larger diameter than placebo polymer fibers except for parts where the cells were wrapped by the matrix indicating that the electrospinning process itself was not influenced by the presence of the biodrugs. This is promising from the aspect of scale-up possibilities, because the scaling up of PVA and PVP nanofiber production technologies are thoroughly investigated [10, 11].
Considering our aims, the most important results are that the biological activity of biodrugs – the viability of bacteria and the activity of enzyme – did not decrease significantly during the whole process. This means that we have succeeded to produce solid polymer nanoweb containing biodrug with a bioactivity nearly 100% compared to that of the initial solvents.
Figure 7. Electrospinning has no impact on activity of biodrugs
Preserving their effectiveness is a fundamental requirement for drugs thus we have investigated how the activity of biodrugs changes in the fibers. After one year, 1 mg of Lactobacillus acidophilus containing sample contained more than 1 million active bacteria, i.e. the effective dose. Remarkable is that after one year, the number of living bacteria decreased only to one third while other papers in this topic using lyophilization [4, 12] have reported a decrease of one or more orders of magnitude during less than a year. These exceptionally good results might be attributable to the gentle milieu of drying and the protective shield of polymer formed around the bacteria during the spinning. The activity of the enzymes has not decreased either in a one year period and in this case about 20 mg sample contains the effective dose. Consequently, the polymer fibers produced in these experiments contain biodrugs in quantity capable of preserving their biological activity on the long term.
A further criterion of applicability of this method is the possibility of scaling-up. The equipment developed by our research group is capable of increasing the spinning capacity in a mechanic way by supplying auxiliary air, which increases production volume 120-fold. During the scale-up, the technology remained gentle as the activity of model agents was not reduced after drying. Our researches indicate that electrospinning is not only a real alternative of traditional drying techniques but it provides solution for several challenges of pharmaceutical technologies of biodrugs.
Expected impact and further research
The results of our experiments show that electrospinning enables production of solid polymer fibers containing stable biodrugs in a much more gentle way than traditional drying processes.
Our further plans include connecting continuous spinning with fermentation, thus developing a technology that is continuous from the beginning to the end. Such a technology, under appropriate regulation can provide consistent product quality, which is of great importance in pharmaceutical industry.
Furthermore, we plan to investigate the testing of other biodrugs also representing high therapeutic importance especially protein medicines that have a growing importance (e.g. monoclonal antibodies).
Publications, References, and Links
Publications
I. I. Wagner, Nagy Z. K., Á. Suhajda, H. Pataki, T. Vigh, P. L. Sóti, A. H. Harasztos, Gy. Marosi, New Method for Preparation of Tablets Containing Lactobacillus acidophilus, Periodica Polytechnica, accepted on: 19. 05. 2014., if: 0,269
II. A. Balogh, G. Drávavölgyi, K. Faragó, A. Farkas, T. Vigh, P. L. Sóti, I. Wagner, J. Madarasz, H. Pataki, Gy. Marosi, Zs. K. Nagy, Plasticized drug-loaded melt electrospun polymer mats: characterization, thermal degradation and release kinetics, Journal of Pharmaceutical Sciences, 103, 1278-1287, (2014)
III. I. Wagner, Nagy Z. K., Á. Suhajda, T. Tobak, A.H. Harasztos, T. Vigh, P. L. Sóti, H. Pataki, K. Molnár, Gy. Marosi, Nanofibrous solid dosage form of living bacteria prepared by electrospinning, 8, 352-361, (2014) if.: 2,294
IV. I. Wagner, H. Pataki, A. Balogh, Z.K. Nagy, A. H. Harasztos, Á. Suhajda, G. Marosi, Electrospun nanofibers for topical drug delivery, European Journal of Pharmaceutical Sciences, 44, Suppl. 1: 148 (2011) if: 3,291
V. Z.K. Nagy, A. Balogh, I. Wagner, P. Sóti, H. Pataki, K. Molnár, G. Marosi. Nanofibrous drug delivery systems for enhanced dissolution prepared by electrospinning, European Journal of Pharmaceutical Sciences, 44, Suppl. 1: 152-153 (2011) if: 3,291
VI. Z.K. Nagy, K. Nyúl, I. Wagner, K. Molnár, G. Marosi, Electrospun water soluble polymer mat for ultrafast release of Donepezil HCl, Express Polymer Letters 4: 763–772 (2010) if: 1,575
Links
References
[1] D.A. Parkins, U.T. Lashmar. The formulation of biopharmaceutical products. Pharmaceutical science & technology today. 3:129-137 (2000)
[2] Y.F. Maa, S.J. Prestrelski. Biopharmaceutical powders particle formation and formulation considerations. Current Pharmaceutical Biotechnology. 1:283-302 (2000)
[3] S.M. Patel, M.J. Pikal. Emerging Freeze-Drying Process Development and Scale-up Issues. AAPS PharmSciTech. 12:372-378 (2011)
[4] C. Morgan, N. Herman, P. White, G. Vesey. Preservation of micro-organisms by drying; a review. Journal of microbiological methods. 66:183-193 (2006)
[5] C. Ratti. Hot air and freeze-drying of high-value foods: a review. Journal of Food Engineering. 49:311-319 (2001)
[6] S. Rudy. Energy consumption in the freeze- and convection-drying of garlic. TEKA Kom Mot Energ Roln - OL PAN. 9:259-266 (2009)
[7] J.E. Allsworthand J.F. Peipert. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstetrics & Gynecology. 109:114 (2007)
[8] D.M. Paige, Lactose Intolerance, Encyclopedia of Human Nutrition (Third Edition), Pages 67–73 (2013)
[9] J. Doshi, D.H. Reneker. Electrospinning process and applications of electrospun fibers. Journal of electrostatics. 35:151-160 (1995)
[10] G. Zayed, Y. H. Roos. Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochemistry 39: 1081-1086
