|
|
BMe Research Grant |
|
Doctoral School of Physics
Department of Physics/Institute of Physics
Supervisor: Dr. Kézsmárki István
The Magneto-Optical Diagnosis of Malaria
Introducing the research area
Malaria is still one of the deadliest infectious diseases in the world, despite the fact that it is curable by proper drug treatment. However, the key to a successful treatment is the early and accurate diagnosis, which is often inaccessible in the endemic developing countries due to the lack of professional or financial resources. The main goal of my research is to develop a diagnostic device based on magneto-optical detection principles that is suitable for the sensitive and cost-effective diagnosis of malaria.
Brief introduction of the research site
I’m a member of the research team of the Magneto-optical Spectroscopy Laboratory at the Department of Physics, BME. The main scientific profile of our group is the application of magneto-optical spectroscopic methods to reveal the exotic magnetic and optical properties of novel materials, as well as utilizing them for biology-related aims. We have started the development of the malaria diagnostic instrument (RMOD) in this laboratory, whereas I performed the measurements on parasite cell cultures in malaria research laboratories at the IMM in Portugal and the UWA in Australia.
History and context of the research
Malaria infection is a major issue in global healthcare, threatening almost half of the world’s population, imposing serious human and financial sacrifices even today. According to the recent report of WHO, in 2014 there have been 200 million registered infections and 584 thousand casualties worldwide. Consequently, the fight against malaria is a global strategic goal of high priority. One of the most important aims of the proposal accepted in 2015 – besides prevention programs and supporting drug and vaccine development [1] – is providing a full-scale accessibility of diagnostics in the poorest and most threatened areas. The key to achieve this goal is, however, a cost-effective, automatized diagnostic procedure, demanding no expertise, which allows the sensitive but cheap detection of the disease. The diagnostic methods available today either require expertise (microscopy) or the cost of a single diagnosis is too high (rapid diagnostic tests - RDT).
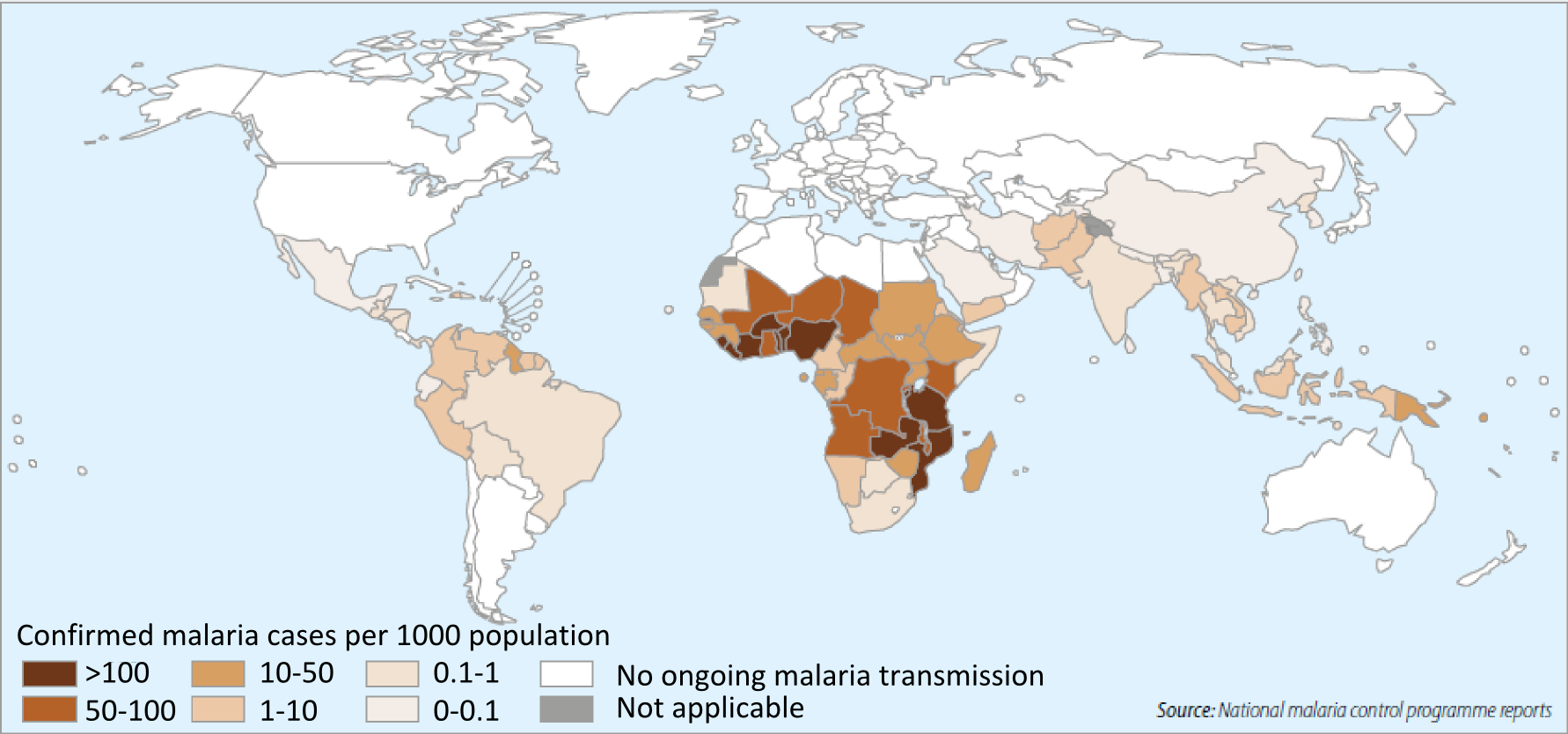
Figure 1. The malaria endemic regions of the Globe (WHO, 2014)
Our group is developing a diagnostic methodology for the detection of malaria parasites in infected blood samples that relies on purely physical principles requiring no biochemical reagents. The target of the method is hemozoin, a by-product of the infection. Hemozoin is produced in the blood-stage of the infection [2], and released to the bloodstream of the patient simultaneously with the occurrence of the first symptoms. Hemozoin is a unique indicator of the disease and is always present during the blood stage of the infection. Furthermore, it has specific magnetic and optical properties [3], making it an ideal diagnostic target. Recently, a several research groups have proposed its use as a biomarker of the infection [4-8].
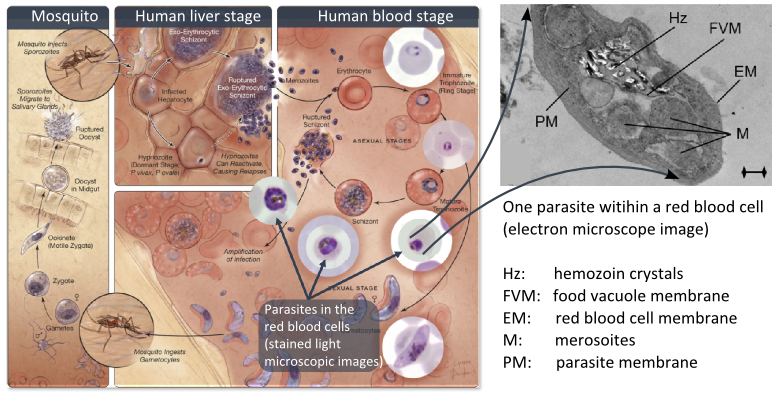
Figure 2. Left: The complex life cycle of the malaria parasites. Right: Hemozoin crystals within the food vacuole of a parasite (electron microscope image).
The aim of the research, questions raised
The primary aim of my research is the development of a malaria diagnostic device that is sensitive enough to detect an early-stage infection when the concentration of hemozoin is extremely low. At the same time, the device should be cost-effective and easy-to-use, making it suitable for in-field diagnostics.
First, I assessed the feasibility of the magneto-optical detection of hemozoin using laboratory-grade instruments. In order to optimize the physical parameters (strength of the magnetic field, wavelength of the applied laser light) for the construction of a portable and cheap device, I investigated the anisotropic optical and magnetic properties of hemozoin [A].
Based on the results of magnetization and optical spectroscopic measurements, I constructed the prototype of the portable device, then I began to validate its performance. In the course of the validation, the proposed steps from the simplest model towards the real field tests were the following:
(i) Feasibility study on the suspension of synthetic hemozoin crystals in blood [A]
(ii) Measurements on in-vitro cell-culture samples of infected red blood cells [B]
(iii) Measurements of blood samples of infected mice [C]
The last grade of the validation, which we propose to perform in the near-future:
(iv) Validation by in-field diagnosis, using infected blood samples of malaria patients.
Besides the development of the instrument, I also aimed at producing important results in basic malaria research. An extensive investigation of the physical properties of hemozoin has been already realized as a product of the device-development [A]. Moreover, the instrument provides a fast and cost-effective alternative to drug susceptibility assessment of antimalarials [B]. Furthermore, our methodology allows the accurate measurement of hemozoin accumulation and clearance rates during the course of the infection, which may raise significant scientific attention [9]. Correspondingly, the performance of large-scale drug tests, and fundamental research concerning hemozoin kinetics are also among my future goals.
Methods
The possible detection of hemozoin by magnetic principles is provided by the iron ion situated in the center of the porphyrin rings – the elementary building blocks of the crystal – is in a high valence, paramagnetic state. It means that it can be magnetized as opposed to the diamagnetic iron occurring naturally in blood.
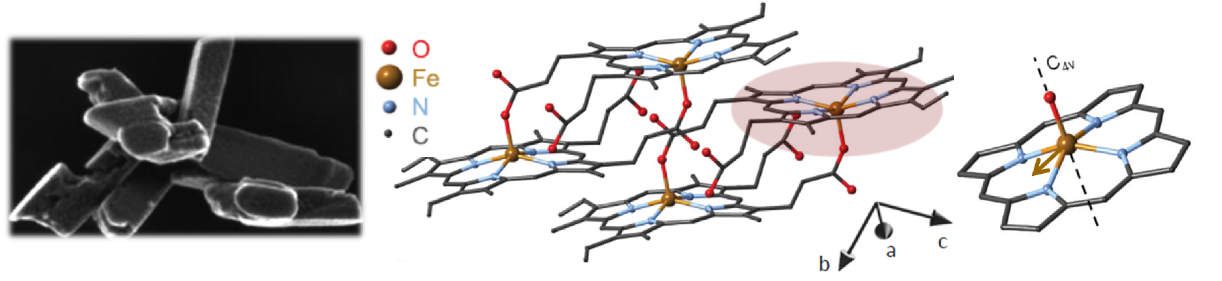
Figure 3. Left: The morphology of hemozoin crystals on an electron microscopic image; Center: two unit cells of the crystal; Right: the porphyrin ring and the magnetic anisotropy axis.
The low symmetry of the crystals gives rise to linear dichroism – that is the variable transmission depending on the polarization direction of incoming light –, which enables their optical detection in blood. In order to superpose the effect of all the individual crystals in the suspension constructively, their optical axes should be co-aligned. The magnetic anisotropy of the crystals enables the alignment of the crystals meaning that the hemozoin crystals are co-aligned via the application of an external magnetic field. Then the probing light with parallel and perpendicular polarization to the magnetic field direction is shone through the sample. The difference in the transmitted intensity of the two different polarizations is measured. The intensity difference is proportional to the quantity of the hemozoin crystals accumulated in the blood, therefore the progress of the infection can be quantified.
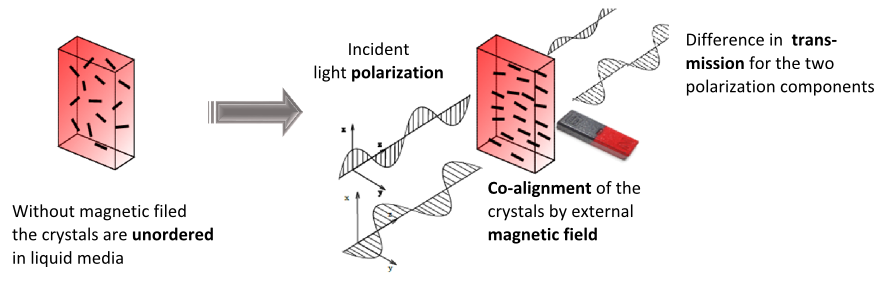
Figure 4. A simple model illustrating the magneto-optical detection of the hemozoin crystals.
For the diagnosis of the infection in its initial stages, the ultra-sensitive detection of hemozoin is needed. Therefore, the application of a modulation methodology is necessary for the realization of the device. The above described measurement principles have been proposed by other research groups before [6], but as an inherent disadvantage of the magnetic field modulation scheme, the device they constructed was too expensive and large-sized to be applied in clinical practice.
The key to our methodology is that we modulate the direction of the magnetic field, instead of its strength. This was realized by the rotation of a magnetic ring consisting of permanent magnets (called Halbach-array). The hemozoin crystals present in the blood follow the rotation of the external magnetic field. The horizontally and vertically polarized components of the polarized light transmitted through the sample change in the opposite phase due to the change in the direction of the hemozoin crystals’ actual alignment. To separate the two orthogonal polarization components a Wollaston-prism was used, and the differential intensity between the two beams was measured by a so-called balanced photo-diode bridge. The realized methodology provides a highly sensitive measurement, which allows the detection of an intensity change of one part to a million, relative to the total intensity, thus quantities of hemozoin as low as 15 pg in a milliliter of blood can be readily detected [A]. Furthermore, the instrument comprises cheap, off-the-shelf optical elements, such as an ordinary laser diode and a permanent magnet, therefore the building costs can be low enough to offer an alternative to the current diagnostic methods. Moreover, the device can be easily scaled down to a size that is easily transportable to be used as an in-field diagnostic setup.
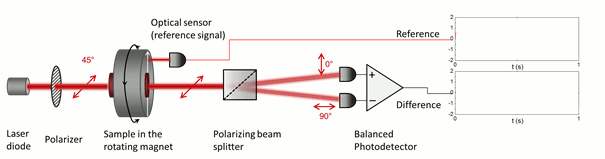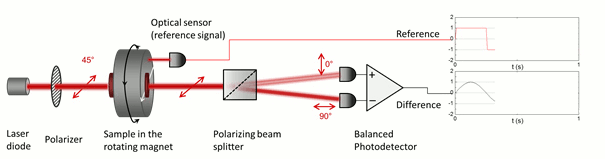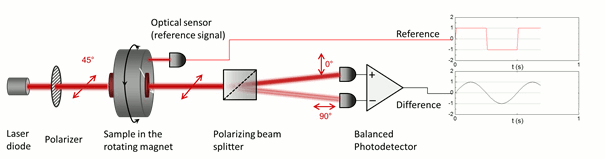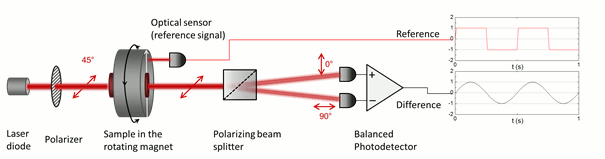
Figure 5. The detection scheme of the rotating-crystal magneto-optical (RMOD) device
Results
In the initial stages of the research I investigated the fundamental physical properties of hemozoin. The magnetization measurements performed on a SQUID underpinned the easy-plane paramagnetic nature of hemozoin [A]. I studied the the degree of the magnetic alignment depending on the strength of the applied field via the magnitude of the magnetically induced linear dichroism. According to the experiments, a moderate magnetic field of 0.5-1 T, achievable by permanent magnets, is sufficient for a complete magnetic alignment. Our experiences are in good agreement with the results of other groups measured by magnetic resonance spectroscopy [10], and moreover, they provide a step forward from earlier ideas about the nature of the magnetic alignment of the crystals [6].
Magnetically induced linear dichroism spectrum of the crystals was investigated by a spectrometer assembled in our laboratory, applying polarization modulation methodology. Based on the spectral position of the linear dichroism peaks and the wavelength dependence of the transmission in blood, the optimal wavelength of the probing laser light was found to be in the vicinity of 680 nm.
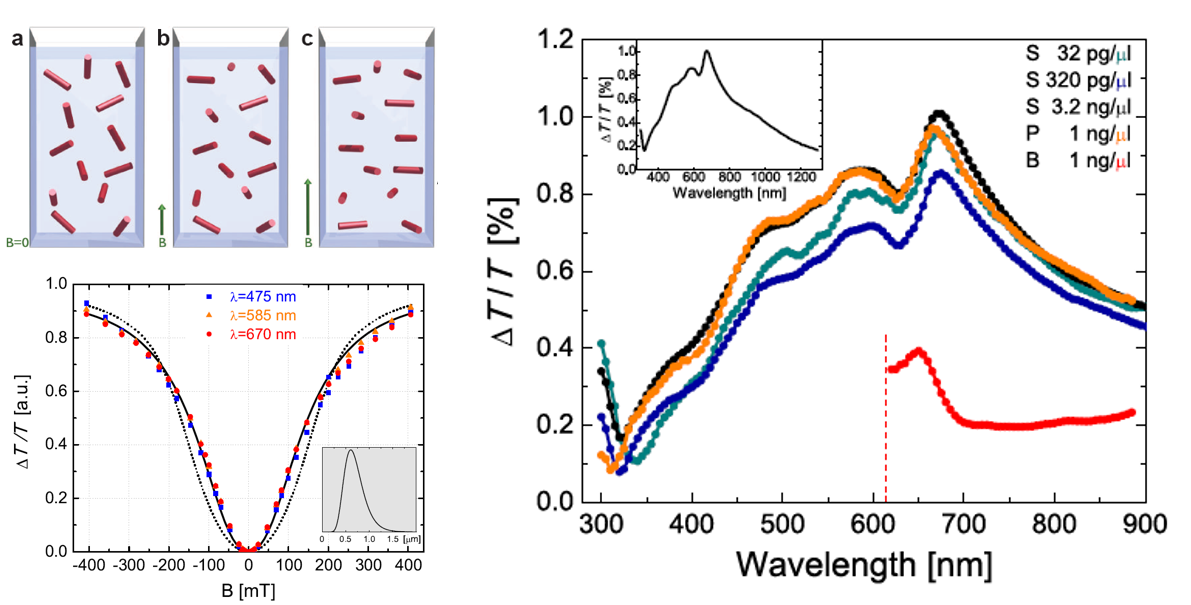
Figure 6. Left: The magnetic field dependence of the crystal-ordering in suspensions. Up: the orientation of the magnetic hard axes’ of the crystals in increasing magnetic field. Bottom: the magnitude of the linear dichroism signal as a function of magnetic-field strength, ie. the degree of crystal-ordering Right: linear dichroism spectra of hemozoin suspensions (S: saline, P: blood plasma, B: whole blood)
As a first step of the validation, measurements were completed on the aqueous suspensions of synthetic β-hematin crystals (the synthetic equivalent of hemozoin) [11]. The detection threshold of the synthetic crystals suspended in water fell in the 0.5-1 pg/µl range, whereas this value was found to be ~10 pg/µl due to the increased absorption and light scattering in blood. These concentration values indicated – through comparison with the estimated concentration of hemozoin in the bloodstream of infected patients – that the detection threshold of our device exceeds that of the rapid tests, and is within the range of microscopic diagnosis, thus the simplest validation step proved to be successful [A].
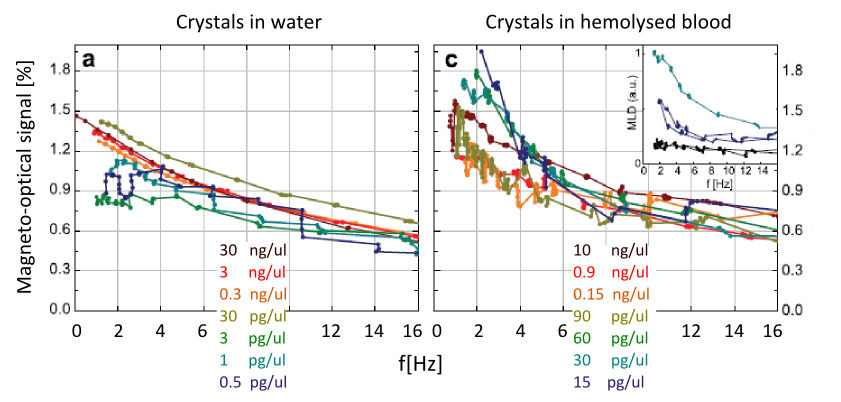
Figure 7. The magneto-optical signals of synthetic crystal suspensions as a function of the rotational speed of the magnetic field. The curves of different crystal concentrations have been normalized with their nominal concentration values to demonstrate the linear concentration dependence.
In the second phase of the validation of the device, red blood cell culture samples infected with P. falciparum parasites were investigated, provided by the malaria research institute of WEHI. Blood samples were prepared in different maturation stages of the parasites as representatives of the different Plasmodium species found naturally in human blood. According to the expectations, the measured magneto-optical signal was found to be proportional to the concentration of parasites over a wide range, and it also correlated positively with the maturation stage. The detection threshold of the parasites was 0.0008% for early ring cultures, and it was 0.0002% for later schizont stages [B]. These values indicate the possibility of a very early-stage diagnosis of the infection.
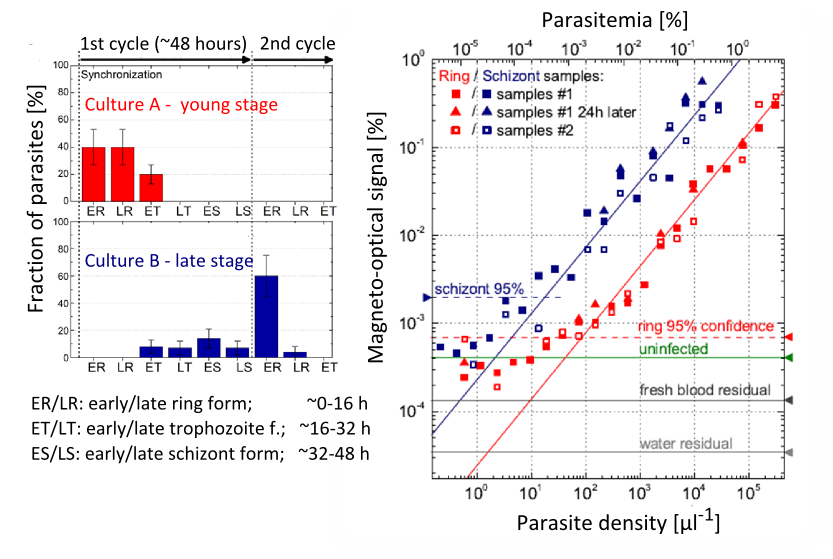
Figure 8. Left: the maturation-stage distribution of the parasites in the originals of the studied culture samples (A and B). Right: magneto-optical signal magnitudes of the 2-fold dilution series of samples A and B. The 95% confidence lines represent the lowest positively detectable parasitemia values.
As the third step of the diagnosis, we aimed at an accurate modeling of the real diagnostic situation by performing mice experiments. The mice were infected by mosquito-borne parasite forms (sporozoites), and then blood samples were drawn from them at specific times. The blood samples were tested by different diagnostic procedures to assess the time of the earliest positive diagnosis for each method. Even though the magneto-optical methodology did not achieve the performance of the ultra-sensitive, but rather expensive PCR, it well exceeded that of the flow-cytometry and the microscopy, which is currently the gold standard of malaria diagnostics. Performing experiments during treatment, we established, that our methodology is able to detect the reinfection of cured mice after a few days clearance period, fulfilling thus one of the essential requirements towards diagnostic methods under development.
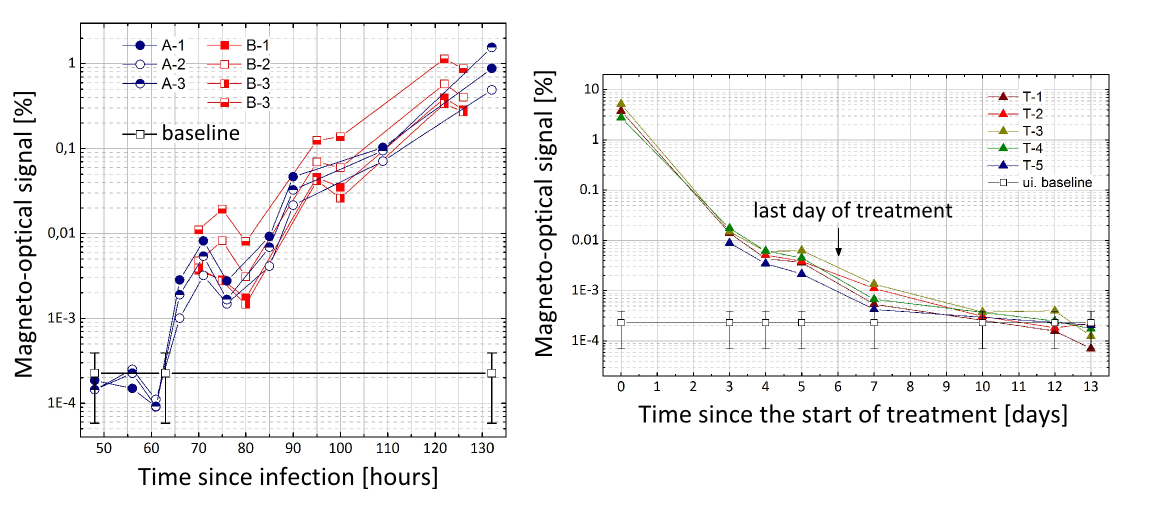
Figure 9. Left: The RMOD signals of seven infected mice during the onset and progression of malaria blood stage infection. Right: RMOD signals of five mice during and after successful treatment of the infection.
Setting up a prototype of the diagnostic device in the IMM in Lisbon, we tested the performance of the device on living, in-vitro maintained cell cultures. Our results show that the magneto-optical device is able to monitor the maturation of the parasites with high sensitivity through the detection of the increasing amount of hemozoin produced. Owing to that, besides its possible diagnostic use, the instrument is readily applicable to perform drug-susceptibility tests of antimalarials [B].
Impact and future research
The development of the RMOD malaria diagnostic device has entered into its final validation stage. The first prototype of the device has been successfully tested on all the model systems of malaria that can be performed under laboratory conditions, ranging from the simplest synthetic samples to in-vivo mice experiments. Our preliminary results indicate that the device can be utilized to detect other anisotropic magnetic particles as well [12].
Our published results induced significant press coverage and brought about cooperative industrial and academic partnerships with prestigious malaria research institutes. Thanks to these cooperations we are able to continue the development of the device in two directions: (i) our colleagues in the IMM institute in Lisbon have started performing large-scale drug tests on parasite cultures, using our prototype; (ii) on the other hand we plan to perform field tests in endemic areas (India and Thailand) in autumn, 2015.
Publications, references, links
Publications
[A] Butykai A, Orbán A, Kocsis V, Szaller D, Bordács S, Tátrai-Szekeres E, Kiss LF, Bóta A, Vértessy BG, Zelles T and Kézsmárki I: Malaria pigment crystals as magnetic microrotors: Key for high sensitivity diagnosis. SCIENTIFIC REPORTS 3: Paper 1431. 10 p. (2013)
[B] Orbán A, Butykai A, Molnar A, Prohle Z, Fulop G, Zelles T, Forsyth W, Hill D, Muller I, Schofield L, Rebelo M, Hanscheid T, Karl S and Kezsmarki I: Evaluation of a novel magneto-optical method for the detection of malaria parasites. PLOS ONE 9:(5) p. e96981. (2014)
[C] Orbán A, Rebelo M, Albuquerque IS, Butykai A, Kezsmarki I and Hänscheid, T: Efficient monitoring of bloodstage infection in a malaria rodent model by the rotating crystal magneto-optical method http://xxx.lanl.gov/abs/1505.07792 (2015)
International media coverage on the method
Physics World, 2012
MIT Technology Review, 2012
Elsevier’s Global Malaria Resource, 2012
References
[1] Noedl H, Wongsrichanalai C, Wernsdorfer WH: Malaria drug-sensitivity testing: new assays, new perspectives. Trends Parasitol. 2003, 19(4):175-81.
[2] Francis SE, Sullivan DJ, Goldberg DE: Hemoglobin metabolism in the malaria parasite plasmodium falciparum. Annu Rev Microbiol. 1997, 51:97–123.
[3] Egan TJ: Physico-chemical aspects of hemozoin (malaria pigment) structure and formation. J of Inorg Biochem. 2002, 91:19–26
[4] Rebelo M, Shapiro HM, Amaral T, Melo-Cristino J, Hänscheid T: Haemozoin detection in infected erythrocytes for Plasmodium falciparum malaria diagnosis—Prospects and limitations. Acta Trop. 2012, 123:58– 61
[5] Karl S, David M, Moore L, Grimberg BT, Michon P, Mueller I, Zborowski M Zimmerman PA: Enhanced detection of gametocytes by magnetic deposition microscopy predicts higher potential for plasmodium falciparum transmission. Malar J. 2008, 7:66.
[6] Newman DM, Heptinstall J, Matelon RJ, Savage L, Wears ML, Beddow J, Cox M, Schallig HD, Mens PF: A magneto-optic route toward the in vivo diagnosis of malaria: preliminary results and preclinical trial data. Biophys. J. 2008, 95:994–1000.
[7] Mens PF, Matelon RJ, Nour BYM, Newman DM, Schallig H: Laboratory evaluation on the sensitivity and specificity of a novel and rapid detection method for malaria diagnosis based on magneto-optical technology (MOT). Malar J. 2010, 9:207.
[8] Lukianova-Hleb EY, Campbell KM, Constantinou PE, Braam J, Olson JS, Ware RW, Sullivan DJ Jr, Lapotko DO: Hemozoin-generated vapor nanobubbles for transdermal reagent- and needle-free detection of malaria. Proc Nat Acad Sci USA 2013, 11:900-905.
[9] Day NPJ, Pham TD, Phan TL et al., “Clearance kinetics of parasites and pigment-containing leukocytes in severe malaria,” Blood 1996, 88(12):4694–4700.
[10] Sienkiewicz, A, Krzystek J, Vileno B, Chatain G, Kosar AJ, Bohle DS, Forró L: Multi-frequency high-field EPR study of iron centers in malarial pigments. J. Am. Chem. Soc. 2006,128:4534–4535.
[11] Egan TJ, Hempelmann E, Mavuso WW: Characterisation of synthetic b-haematin and effects of the antimalarial drugs quinidine, halofantrine, desbutylhalofantrine and efloquine on its formation. J. Inorg. Biochem. 1999, 73:101–107.
[12] Ceolin G, Orban A, Kocsis V, Gyurcsanyi RE, Kezsmarki I and Horvath V: Electrochemical template synthesis of protein imprinted magnetic polymer microrods. JOURNAL OF MATERIALS SCIENCE 48:(15) pp. 52095218. (2013)

