|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Institute of Technical Physics and Materials Science – Centre for Energy Research
Supervisor: Dr.Deák András
Directed Self-assembly of Gold Nanoparticles
Introducing the research area
During my work, I investigated the possibilities of organizing gold nanoparticles into higher-order structures by fine-tuned and pre-designed colloidal interactions governing the self-assembly processes. Gold nanoparticles have special, advantageous optical properties, however, self-assembled nanostructures broaden the range of their field of applications.
Introduction of the research group
This project was done in the Institute of Technical Physics and Materials Science (MFA), a member of Centre for Energy Research. Our laboratory called Chemical Nanostructures Lab is a joint research laboratory of MFA and BUTE Department of Physical Chemistry and Materials Science. The main topics are wet chemical synthesis and optical characterization of plasmonic nanoparticles, nanostructured surfaces and nanoassemblies. Current work is a part of a European Union’s Seventh Framework Project (UNION – Ultra Versatile Nanoparticle Integration Into Organized Nanoclusters).
History of the research and its broader context
The special optical properties of gold nanoparticles (NPs) are based on the localized surface plasmon resonance (LSPR) phenomenon [1]. When the particles interact with the incident light, the conduction electrons start oscillating collectively. This process becomes resonant, if the frequency of oscillation and the incident light are equal. Besides the absorption, gold nanoparticles also scatter the light, therefore the sum of absorption and scattering, which is called extinction, can be measured. For isotropic particles (spheres) the extinction spectrum contains only one LSPR peak, however, if the symmetry breaks (e.g. nanorods), new peaks evolve due to the different oscillation directions. In the vicinity of the particles (i.e. near-field), significant enhancement of the incident field can evolve [2]. If two or more particles are getting closer to one another, their near-fields overlap. This plasmon coupling phenomenon changes the optical properties of these assembled structures compared to the individual nanoparticles [3]. Non-Raman active molecules localized in the confined space between the particles can be changed to Raman active and can provide enhanced Raman response. Consequently, investigation of controlled, externally triggered self-assembly routes is of great importance in order to build nanoparticle assemblies with internal order. This requires the synthesis of appropriately designed and surface-modified nanoparticles and the modulation of the relevant colloidal interactions as well.
Aim of the research, research questions
Besides the two basic colloidal interactions (attractive van der Waals and electric double layer repulsion), optimal self-assembly process requires a third interaction to be involved: the steric repulsion. This steric interaction originates from the presence of surface attached macromolecules on the nanoparticle surface. The total colloidal interaction is the sum of these three main interactions and can be modulated through the steric component, if the thickness of the polymer layer (the length of the polymer chains) can be tuned by external trigger. This allows developing a suitable potential curve with moderate attraction (few kT) in order to prepare compact nanoparticle clusters [4]. One of the main research question was: is it possible to trigger the length of surface attached PEG (poly(ethylene glycol)) molecules? The lower critical solution temperature of PEG is well above 100°C in aqueous solutions [5], however, in the presence of ions, this temperature decreases (even up to 40°C) [6]. I targeted to exploit the steric interaction modulation based on the structural changes of polymer chains in order to tune the overall colloidal interaction. My aim was to demonstrate through colloidal interaction calculations and self-assembly experiments, that this approach is suitable for the preparation of nanoparticle clusters. Furthermore, investigation of the effect of control parameters on the kinetics of assembly and on the structure of the resulted clusters was also targeted. Surface modified gold nanoparticles are extremely stable in aqueous solution (if the solution does not contain salt at room temperature). Consequently, my aim was to use them as tracer objects in capillary lithographic experiments and to prepare two-dimensional array of nanoparticle rings with tunable internal diameter arranging the nanoparticles underneath (sub)microparticles.
Methods
Gold nanoparticles were synthesized by the traditional Turkevich method, where aqueous solution of gold salt was heated to boil with Na-citrate. This method provides 18 nm gold NPs with narrow size distribution, that can be grown to NPs of 40, 45 and 60 nm in diameter in the presence of Au(III) ions and citrate anions. The surface of gold NP can be effectively modified by molecules containing sulphur and nitrogen atoms. I used α-methoxy-ω-mercaptopoly(ethylene glycol) and α-methoxy-ω-aminopoly(ethylene glycol) as stabilizers. The optical properties and size of assemblies as a function of temperature and time were investigated by changing the temperature of responsive polymer-coated NPs solution at increased ionic strength systematically. Due to the special optical properties of gold nanoparticles mentioned in the introduction, aggregation and clustering can be followed by spectroscopy in the visible wavelength range sensitively [S4]. Aggregation manifests itself in the redshift or broadening of LSPR peak and in the evolution of new peak(s) due to plasmon coupling. Cluster size evolution was followed by dynamic light scattering (DLS), cluster structure was investigated by electron-microscopy (SEM, TEM). Time-dependent spectroscopy and DLS allowed me interpreting the effect of control parameters (salt concentration and temperature) on the kinetics of the aggregation and the structure of assemblies.
Organizing gold NPs into ring-shaped structures was carried out by capillary lithography. Firstly, I investigated the wetting properties of materials relevant in the experiments, which gave information about the affinity of spontaneous adsorption of gold nanoparticles at these interfaces. Secondly, sacrificial Langmuir-Blodgett template mono-layers [8] were prepared from polystyrene micro-spheres on Si substrate. Then nanoparticle sol was dried on different template mono-layers in controlled way. Evaporation of solvent induced the formation of gold nanoparticle rings by constraining the NPs into the wedge between PS template particles and substrate. The 2D pattern of nanorings can be developed by removing of sacrificial template mono-layer. The location of NPs after drying provides information about the stages of dewetting and about the effect of template layer defects on the evolved structure. Periodic, hexagonal pattern of nanoparticle rings originates in the ordering of template mono-layer. I confirmed, that internal diameter of rings can be tuned by changing the size of the nanoparticles and template particles precisely. Application potential of ring pattern as surface enhanced Raman spectroscopy (SERS) substrate were investigated by performing micro-Raman spectroscopy using mercaptobenzoic acid as reporter molecule.
Results
Preparation of compact nanoparticle clusters by fine-tuning colloidal interactions [S1, S2]
Thickness of polymer brushes on the nanoparticles were estimated from hydrodynamic sizes measured by DLS. Assuming the chain collapse of surface attached polymers, colloidal interactions were calculated for different brush thicknesses. Results are shown in Figure 1 for 18 and 40 nm particles.
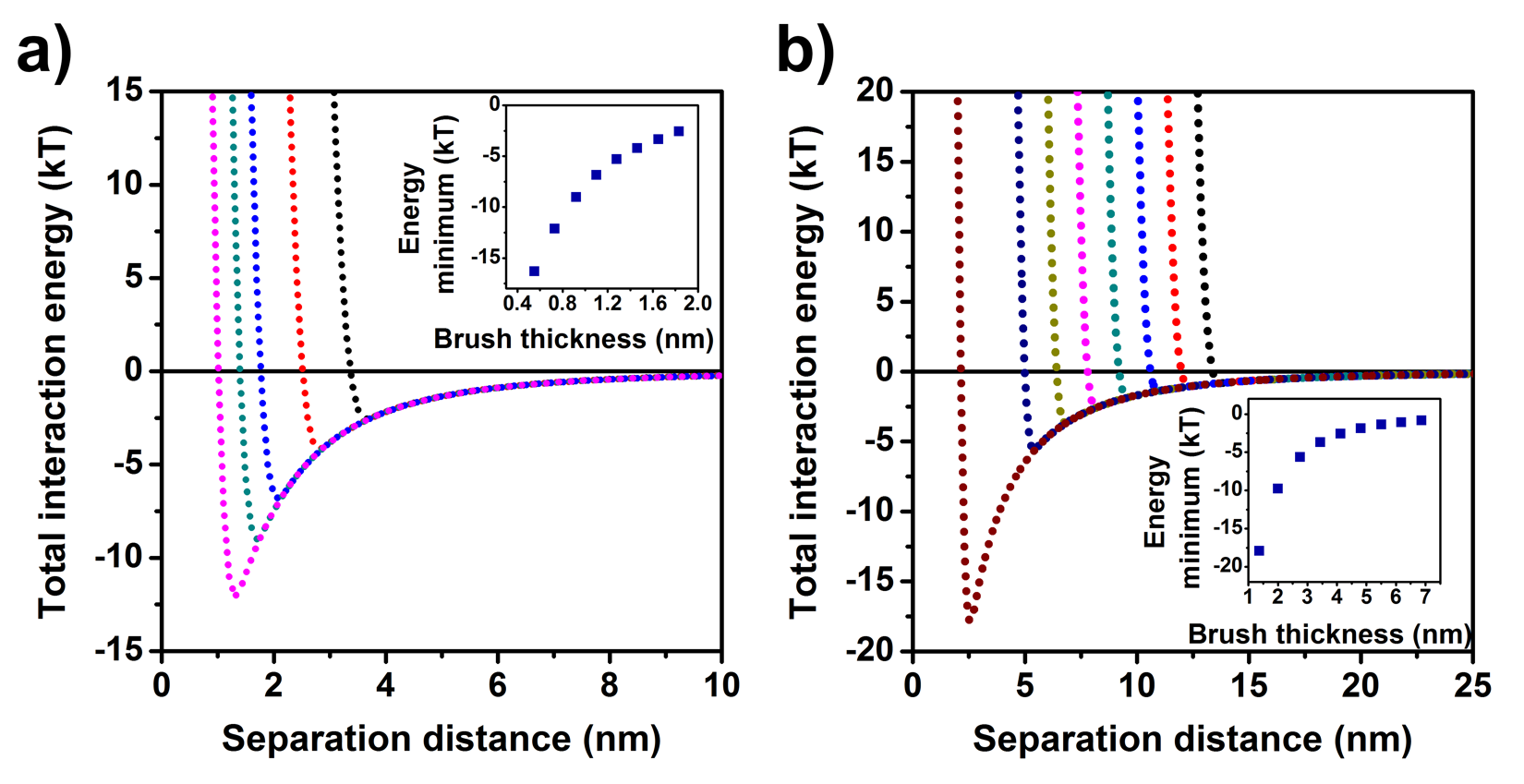
Figure 1. Calculated total nanoparticle-nanoparticle interaction potentials as a function of mPEG brush thickness for (a) AuNP(18nm)@mPEG-SH(750) and (b) AuNP(40nm)@mPEG-SH(2000) at 60°C. Insets show the energy minima for different brush thicknesses.
Calculations showed, that PEG chain collapse and the coupled steric interaction changing can develop significant driving force for clustering of gold NPs (attractive potential of few kT [4]). In addition, increased electrolyte concentration and temperature can be used as external triggers to induce self-assembly. Clustering seemed to be feasible according to the calculations, however, I aimed at confirming this theory experimentally as well. DLS measurements were carried out as a function of electrolyte concentration and temperature in order to prepare DLS contour plots for better visualization of size changes (Figure 2).
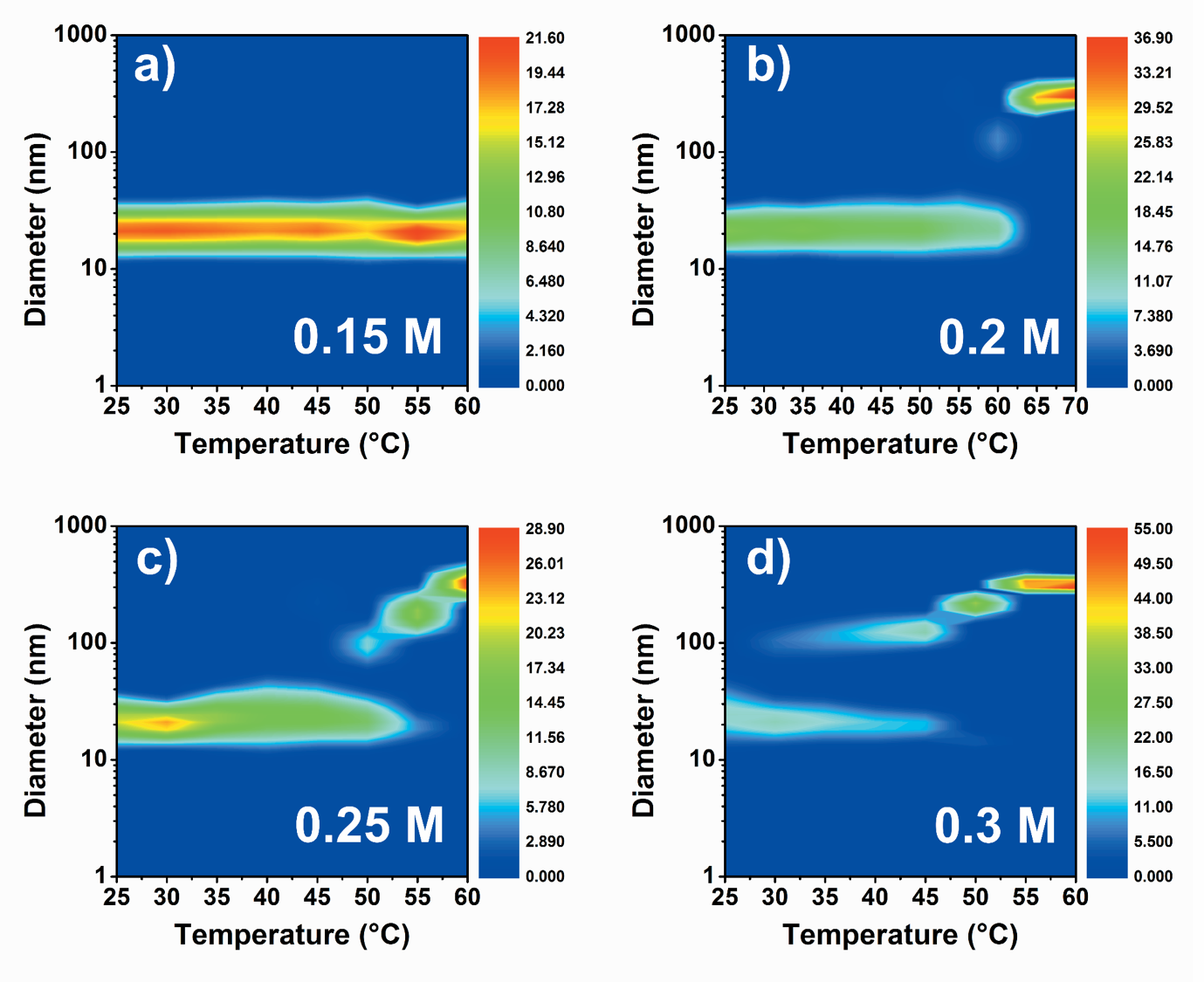
Figure 2. DLS contour plots for AuNP(18nm)@mPEG-SH(750) system at different K2SO4 concentrations: (a) 0.15 M, (b) 0.20 M, (c) 0.25 M and (d) 0.30 M. Color bar represents the DLS intensity.
At 0.15 M, size increase cannot be observed, however, aggregation of nanoparticle starts at higher salt concentrations. Threshold temperatures of aggregation decrease at increased electrolyte concentration. Self-assembly of particles can be followed by VIS spectroscopy: new coupled mode evolves in the extinction spectrum above the threshold temperature in time (Figure 3).
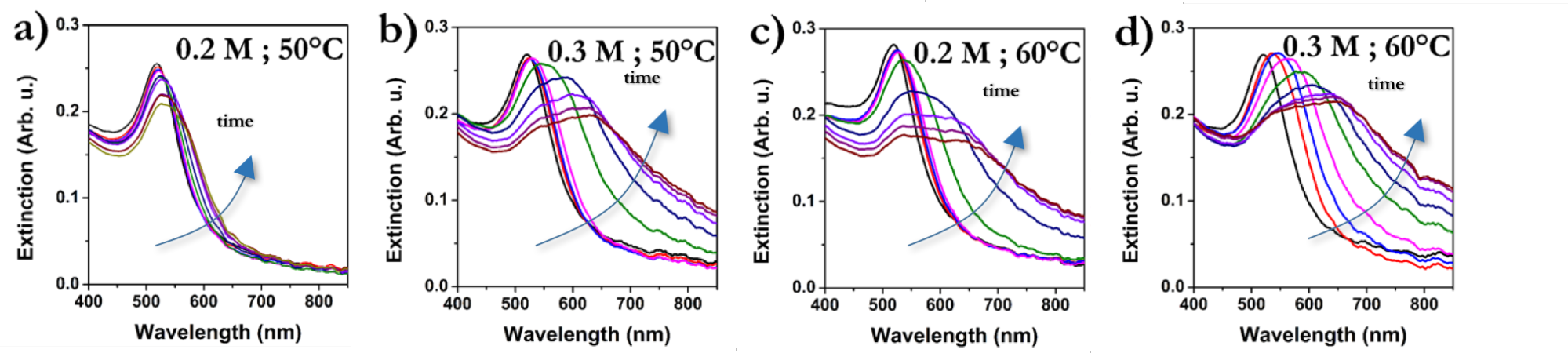
Figure 3. Evolution of extinction spectra in time for Au(20nm)@mPEG-NH2(2000) at different salt concentrations and temperatures.
Applied electrolyte concentration and temperature (as two key parameters) have significant effect on the structure of aggregates: at lower salt concentration and temperature, associates containing only few NPs evolve, but at higher concentration and temperature the clusters are larger and compact (Figure 4).
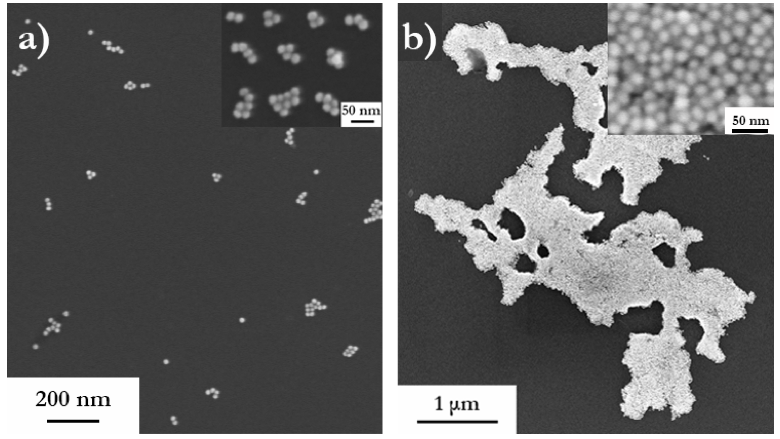
Figure 4. Structure of clusters prepared at different electrolyte concentrations and temperatures: (a) 0.2 M, 50 °C; (b) 0.3 M, 60 °C. Insets show closeups of the structures.
Preparation of single chain gold nanoparticle rings by capillary lithography [S3]
In the last stage of drying of evolved liquid rings underneath the PS particles, gold NPs are constrained to form ring-shaped structures under the template particles on the substrate. Figure 5a–d show nanoparticle rings composed of 45 and 65 nm particles using different PS particle sizes. Internal diameter of nanorings depends on the template diameter and NP diameter as well. Calculated and measured ring radii showed excellent agreement, which demonstrates the tunability of ring geometry (Figure 5e).
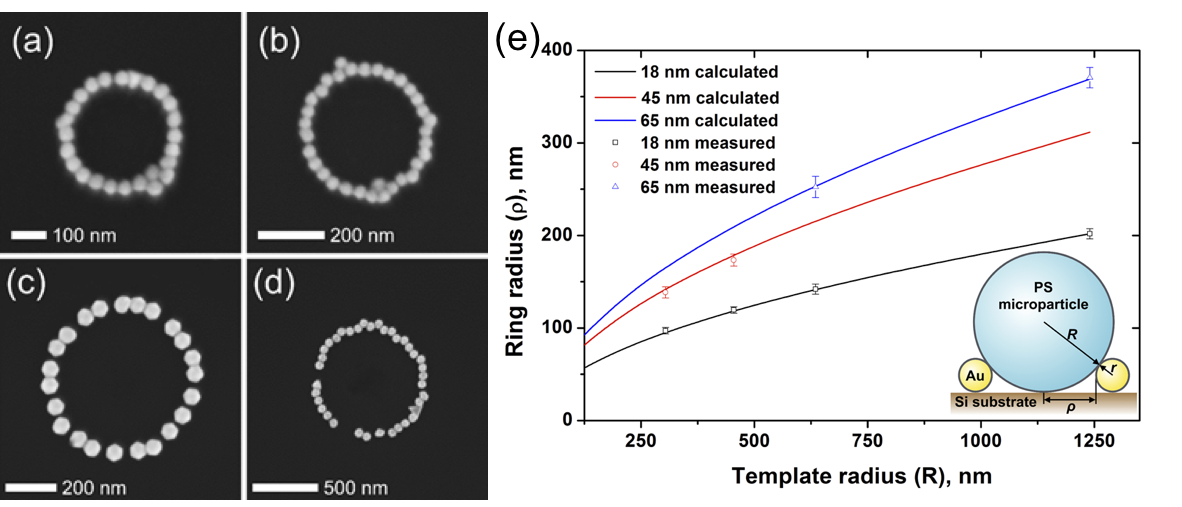
Figure 5. Single chain gold nanoparticle rings prepared from (a,b) 45 nm and (c,d) 65 nm particles using different template particle sizes: (a) 608 nm, (b) 909 nm, (c) 1.27 μm and (d) 2.48 μm. (e) Internal ring radii as a function of template diameter.
Two-dimensional pattern of nanorings can be seen in Figure 6.
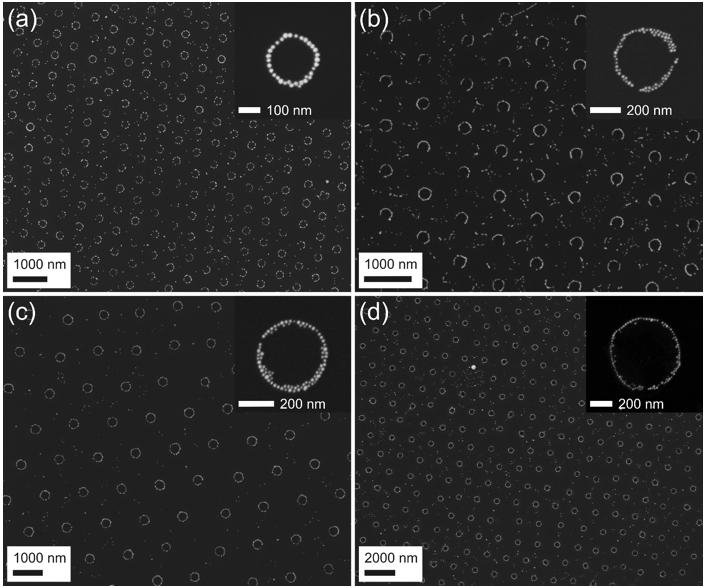
Figure 6. Nanorings from 18 nm gold NPs prepared using PS template particles with diameter of 608 nm (a), 909 nm (b), 1.27 μm (c) and 2.48 μm (d).
Impact of the research, future plans
Higher-order nanoparticle assemblies have application potential in the field of sensorics, theranostics (cancer therapy and diagnostics) and photovoltaics. Solid supported two-dimensional array of gold nanoparticle rings can be used as SERS substrate as we have confirmed. The structure enhances the Raman signal of mercaptobenzoic acid significantly, while from the range without rings Raman signal cannot be detected (Figure 7).
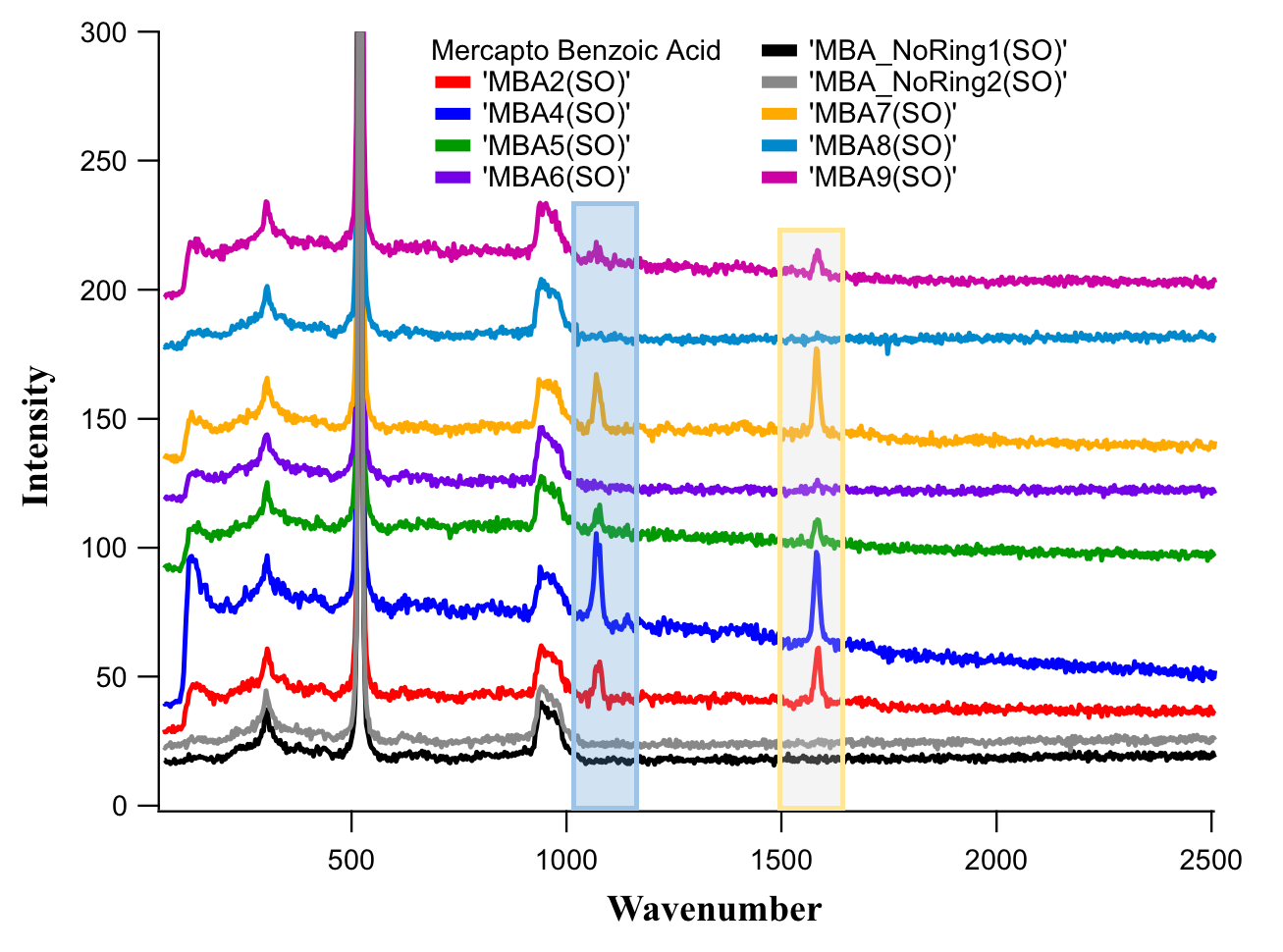
Figure 7. Raman spectra of mercaptobenzoic acid measured from nanoring array (black and gray curves) and from bare substrate (other colors).
Currently, we are working on detecting scattering spectrum of individual nanoparticle rings and comparing the measured optical response to the simulated spectra.
Publications, references, links
List of own publications related this work:
[S1] Zámbó, D.; Radnóczi, G. Z.; Deák, A. Preparation of Compact Nanoparticle Clusters from Polyethylene Glycol-Coated Gold Nanoparticles by Fine-Tuning Colloidal Interactions. Langmuir 2015, 31 (9), 2662–2668. (IF=4.457)
[S2] Zámbó, D.; Pothorszky, S.; Brougham, F. D.; Deák, A. Aggregation Kinetics and Cluster Structure of Amino-PEG Covered Gold Nanoparticles. RSC Advances 2016, 6 (32), 27151–27157. (IF = 3.84)
[S3] Nagy, N.; Zámbó, D.; Pothorszky, S.; Gergely-Fülöp, E.; Deák, A. Identification of Dewetting Stages and Preparation of Single Chain Gold Nanoparticle Rings by Colloidal Lithography. Langmuir 2016, 32 (4), 963–971. (IF = 4.457)
[S4] Zámbó, D.; Deák, A. Optical Simulations of Self-Assembly Relevant Gold Aggregates: A Comparative Study. Periodica Polytechnica Chemical Engineering 2016 (accepted manuscript) (IF=0.296)
Links:
Surface plasmon resonance: https://en.wikipedia.org/wiki/Surface_plasmon_resonance
Turkevich method: https://www.youtube.com/watch?v=EzuRMTe_lho
Langmuir-Blodgett technique: https://www.youtube.com/watch?v=j8yqyRr2VQg
Surface Enhanced Raman Spectroscopy (SERS): https://en.wikipedia.org/wiki/Surface-enhanced_Raman_spectroscopy
References:
[1] https://en.wikipedia.org/wiki/Surface_plasmon_resonance
[2] Chung, T.; Lee, S.-Y.; Song, E. Y.; Chun, H.; Lee, B. Plasmonic Nanostructures for Nano-Scale Bio-Sensing. Sensors 2011, 11 (12), 10907–10929.
[3] Taylor, R. W.; Esteban, R.; Mahajan, S.; Coulston, R.; Scherman, O. A.; Aizpurua, J.; Baumberg, J. J. Simple Composite Dipole Model for the Optical Modes of Strongly-Coupled Plasmonic Nanoparticle Aggregates. J. Phys. Chem. C 2012, 116 (47), 25044–25051.
[4] Klotsa, D.; Jack, R. L. Predicting the Self-Assembly of a Model Colloidal Crystal. Soft Matter 2011, 7 (13), 6294.
[5] Bae, Y. C.; Shim, J. J.; Soane, D. S.; Prausnitz, J. M. Representation of Vapor–liquid and Liquid–liquid Equilibria for Binary Systems Containing Polymers: Applicability of an Extended Flory–Huggins Equation. J. Appl. Polym. Sci. 1993, 47 (7), 1193–1206.
[6] Yen, D. R.; Raghavan, S.; Merrill, E. W. Fractional Precipitation of Star Poly(ethylene Oxide). Macromolecules 1996, 29 (27), 8977–8978.
[7] Turkevich, J.; Stevenson, P. C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55.
[8] https://www.youtube.com/watch?v=j8yqyRr2VQg
[9] https://en.wikipedia.org/wiki/Surface-enhanced_Raman_spectroscopy
