|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Budapest University of Technology and Economics/ Department of Organic Chemistry and Technology
Supervisor: Dr. Faigl Ferenc
Dye-sensitized Solar Cell: a Promising Chance for the Future
Introducing the research area
The purpose of our research was to synthesize organic dye molecules containing fluorazone backbone, which were successfully, effectively produced and successfully tested in Dye-Sensitized Solar Cells (DSSC) cooperating with an Italian research team. So we used a new structure with success in DSSC system.
Brief introduction of the research place
In the Chirotechnology and Organometallic Research Group of the Department of Organic Chemistry and Technology at the University of Technology and Economics (Budapest), Dr Ferenc Faigl's work began with the study of the reactions of various aromatic and heteroaromatic compounds with organometallic reagents as well as the synthesis of dye molecules containing a conjugated double bond system. Our group works closely with the Hungarian Academy of Sciences, and the testing of our dyes in solar cells was carried out in co-operation with the Organic Chemistry Institute (CNR-ICCOM) in Florence, Italy.
History and context of the research
Until today, the replacement of non-renewable energy resources through clean and sustainable energy sources is the biggest problem of humanity. With the advance of science, newer and newer technologies are being invented to transform the natural energies into usable and storable forms more efficiently. [1]
Solar energy is one of the most promising natural energy sources that can be utilized in many ways: building passive houses, transforming solar energy into heat or electric energy, and storing them in thermochemical way. The energy available in the Earth’s oil reserves equals to the energy radiated from the Sun to the Earth in just one and a half day. The current one-year energy consumption of the whole humanity could be fully covered by one-hour solar energy output of the Sun. That is why enormous efforts have been made to develop high-efficiency technologies. The best results come from transforming solar energy into electric energy. So a separate industry was formed for the development of photovoltaic (solar) systems, and in 1954 the first silicon-semiconductor solar cell was introduced. Nowadays, solar power plants produce electricity all over the world (Figure 1/a).
The biggest disadvantage of these cells is their high price which is partly due to their energy and high technology intensities, and partly because they only work in sunshine and high efficiency requires appropriate angle of incidence of sun rays. [2]
The research goal, open questions
Researches continued to overcome these disadvantages resulted the so-called third-generation, dye-sensitized solar cells (DSSC). In 1991 Grätzel and O'Regan published a new TiO2 nanoparticle-coated organic rhodium-containing complex, an organic dye molecule (Figure 1/b). [3]


Figure 1/a: Solar power plant on the Andalusian plateau b: Grätzel with the invented solar cell
These cells use synthetically produced compounds to convert the sun's radiation into electrical energy learned through close observation from the nature. Similarly to naturally occurring photosynthesis, where the function of the chlorophyll pigment is responsible for the absorption, a kind of artificial photosynthesis is realized by the organic dye in the solar cell (Figure 2). [4]
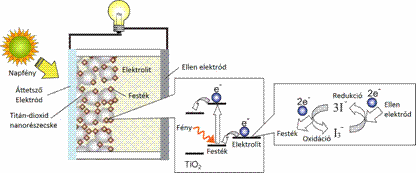
Figure 2: The operation of dye-sensitized solar cells
In the course of solar cells research, metal-free organic dyes appeared additionally to noble metal complexes. They have many advantages: more numerous and more variable structures can be synthesized, their production is cheaper and are accompanied with lower environmental loads, and their molar absorption coefficients are more favourable. Generally, these molecules have so-called D-π-A structures, which means that they have a donor-π-bridge-acceptor system.
Our team, working with an Italian research team, started researching DSSC solar cells. We used computational chemistry methods for the modelling of the structures which may be appropriate in such a system. The dye molecule based on an arylpyrrol backbone designed by quantum chemistry calculations is a donor, a linker serving as a π-bridge and an acceptor part (D-π-A system, Figure 3).
Our research goal was to synthesize the dye and then test the dye in a DSSC system to determine the efficacy of the cell.
Methodology
The compounds were prepared using modern, environmentally friendly, preparative organic chemistry methods. The solvents used for the organometallic reactions were exempted from water and peroxide before use. During the reactions, the inert atmosphere was provided by Schlenk-technique, the reactions were carried out in Schlenk tubes. Dry argon and nitrogen were used as inert gas. Reactions were carried out at 0 °C with ice-water cooling, cooling reactions at -75 °C were solved with an acetone solution of dry ice. The purity of the materials was checked by thin-layer chromatography (TLC), and column chromatography or recrystallization was used to purify the materials. Column chromatographic separations were accomplished using about 20 grams of silica gel (Kieselgel 60 G, Merck) for the purification of about 1 g product. Evaporation was carried out below atmospheric pressure. The structure of the compounds produced in this way was identified by magnetic nuclear resonance spectroscopy (NMR), infrared spectroscopy (IR) and high resolution mass spectrometry (MS).
Optical and electrochemical analysis of the dye molecules was performed using UV/Vis absorption spectroscopy after absorption onto a nanocrystalline TiO2 film. The molecular oxidation potential (Eox) was determined by cyclic voltammetry. The Eox values were determined by averaging the peak potentials of anode and cathode.
To understand the results of optical and electrochemical studies, computer analysis was performed using the Gaussian 09 program package, and the computed values were compared to the measured values.
The dyes produced were tested in a solar cell to sensitize inorganic semiconductor (TiO2), thus absorbing visible light: the photoexcited dye transmits an electron to the semiconductor conduction band.
To assemble the cell, a photoanode was made: we inserted the blocking TiO2 layer onto the leading glass surface (FTO glass). Subsequently, the sensitized electrode was dipped into the solution of the dye. In the case of the counter electrode, a hole was drilled into the glass plate, then filled with electrolyte and the platinum layer was sprayed onto the driver's side and finally heat-treated. Then the cell structure was constructed by inserting the photo and counter electrodes with a SurlynR sealing filler and then filled with iodide / tri-iodide redox pair containing electrolyte through a drilled hole under a vacuum hole.
Results
To synthesize D-π-A compounds electron-rich aromatic amines such as indole or triaryl amine derivatives were found as the best donor units, however, many other heterocyclic units had already been tested. In the π-bridge providing charge-separation, thiophene rings can greatly help to increase efficiency, so this unit is mostly used, but rigid structures containing conjugated double bonding system can also fulfil this role as it can be seen by our structures, too,
It is important for these units to have a rigid structure, because this will ensure trouble-free separation and flow of excited electrons. These structures can be further refined by including "spacer" groups into the molecule, which may reduce undesirable aggregation on the porous surface, provide adequate solubility for the dye and facilitate proper charge separation and electron transport. In the case of the acceptor unit, there are only far fewer solutions: in most cases, cyanoacrylic acid groups have been formed and the compound can be attached to the semiconductor by their acid group, however, some examples can also be found in literature with the so-called dithiofulvenyl units containing other sulphur atoms.
We selected the triarilamine structure for donor unit and a thiophene acrylic ester for acceptor unit. A new synthesis pathway was developed to produce a fluorazone unit carrying the π-bridge function and also further derivatives were synthesized which were organometallically transformed to various indolone and indole derivatives.
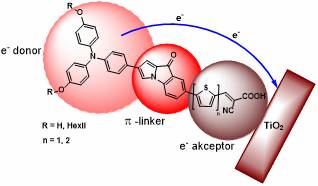
Figure 3: The structure of our organic dyes
Following our synthesis route to synthesize the donor and the acceptor unit, we managed to get in 9 steps with persistent organic preparatory work and high throughput to the target molecules containing the 1-arypyrrole linker unit (Figure 3), which were also tested in dye-sensitized solar cell systems. [H2] Preliminary measurements were very encouraging, in that we achieved higher efficiency than with the reference dye, but in the future we plan to modify the base unit, optimize it and thus increase the efficiency of dyes containing flurazone backbone in Grätzel's solar cells.
Expected impact and further research
Evidence of the benefits of Grätzel cells is the Congress Center in Lausanne, Switzerland itself, where the reception area's wall was covered with DSSCs. They can be positioned vertically, because their efficiency does not depend on the angle of incidence of the solar rays and they provide the complete energy consumption of the building and even reduce the need of using air conditioners due to their shielding effect (Figure 4/a).

Figure 4: A new research facility at the Swiss EPFL Technical University b: A backpack selling by GCell
As an energy source, it is also used for mobiles, backpacks (Figure 4/b) suitable for charging electronic devices, remote controls, tablets, watches, and smoke and position sensors. As these devices can utilize the whole spectra of visible light, they can be used both indoors and outdoors, so every light source might also serve as an energy source.
In our group, further research is in process on this topic; we plan to optimize the linker unit, use another donor unit and thus increase the efficiency of the solar cell built with our dye.
Publications, references, links
Publications
[H1] T. Hergert, B. Varga, B. Mátravölgyi: Life and Science, 2017, 15, 469
[H2] B. Mátravölgyi, T. Hergert, A. Thurner, B. Varga, N. Sangiorgi, R. Bendoni, L. Zani, G. Reginato, M. Calamante, A. Sinicropi, A. Sanson, F. Faigl, A. Mordini: Eur. J. Org. Chem, 2017, 1843
Links
References
[1] Schiermeier, Q.; Tollefson, J.; Scully, T.; Witze, A. and Morton, O: Nature 2008 (454), 816
[2] Saga T: NPG Asia Mater. 2010 (2), 96
[3] O’Regan, B.; Grätzel, M.: Nature 1991 (353), 737.
[4] Mishra, A.; Fischer, M. K. R.; Bäuerle, P.: Angew. Chem. Int. Ed. 2009 (48), 2474
