|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisors: Dr. Grün Alajos és Dr. Keglevich György
The Comprehensive Investigation of the Effect of the Solvents and Reagents in the Synthesis of Bisphosphonic Acid Derivatives
Introducing the research area
In our research, we put in the focus an important and valuable group of P-containing drugs, the α-hydroxymethylenebisphosphonic acid derivatives. More precisely, we wish to improve their synthetic procedure and make it greener. Clarifying the misleading data published and exploration of the reaction mechanism are also an essential part of the research [S1-S10].
Brief introduction of the research place
Our research group (Environmentally-friendly and Organophosphorus Chemical Research Group) operating at the Department of Organic Chemistry and Technology is led by Professor György Keglevich. We are studying the synthesis and utilization of P-containing compounds (P-heterocycles, phosphonic acids, bisphosphonic acids, aminophosphonic acids, hydroxy-phosphonic acids, in transitional metal-complexes useable P-ligands), selective transformations and phase transfer catalyzed reactions under microwave (MW) and solvent-free conditions. Our publications in international journals and the citations confirm the importance of our research achievements.
History and context of the research
The bisphosphonic acid derivatives have two pentavalent tetracoordinate P-atoms, more precisely two phosphonate groups, and a P-C-P moiety, which give them metabolic stability. The pyrophosphoric acid is an important building block of the skeletal system, which structure is similar to the structure of bisphosphonates (Fig. 1). The pyrophosphoric acid has P-O-P moiety instead of P-C-P fraction. Bisphosphonic acids inhibit the elimination of calcium from the bones by binding it to hydroxyapatite crystals [1].
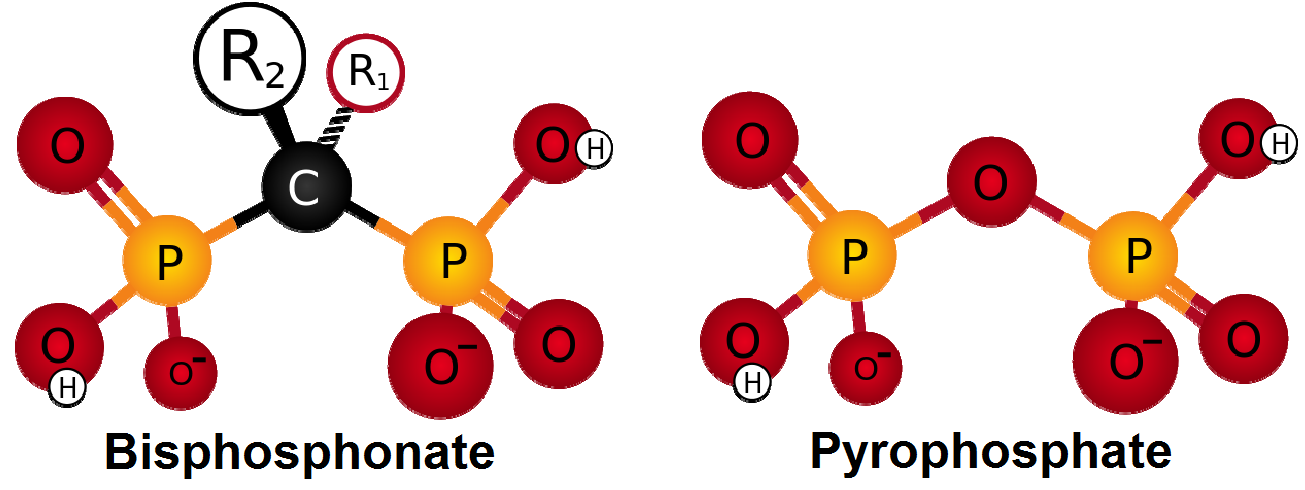
Fig. 1: Structural similarity of bisphosphonic acids and pyrophosphoric acid.
Among the bisphosphonates there are many biologically active species. In the beginning, the bisphosphonates was used in the treatment of tumor diseases affecting bone tissue. Nowadays, they are considered the best drugs in the treatment of osteoporosis (Fig. 2), Paget-disease and tumor-induced hypercalcaemia, but they also have direct anti-cancer (breast, prostate, and kidney) and anti-parasitic effect. Interest is increasing for bisphosphonates, thus the investigation of their syntheses is an important research field [2-5].
Fig. 2: Differences between a healthy and osteoporotic bone tissue.
The bisphosphonic acid derivatives differ from each other in the substitution pattern of the central carbon atom. One substituent of them can be hydrogen or chlorine atom, but the so-called dronic acid derivatives contain a hydroxy group. According to the other substituent, three big groups can be distinguished. The first generation of dronic derivatives does not bear a nitrogen atom in the C-substituent. The representatives of the second and third generations have aminoalkyl or N-heterocyclic substituents, respectively. The biological activity is influenced significantly by the substituents [2,3].
The research goal, open questions
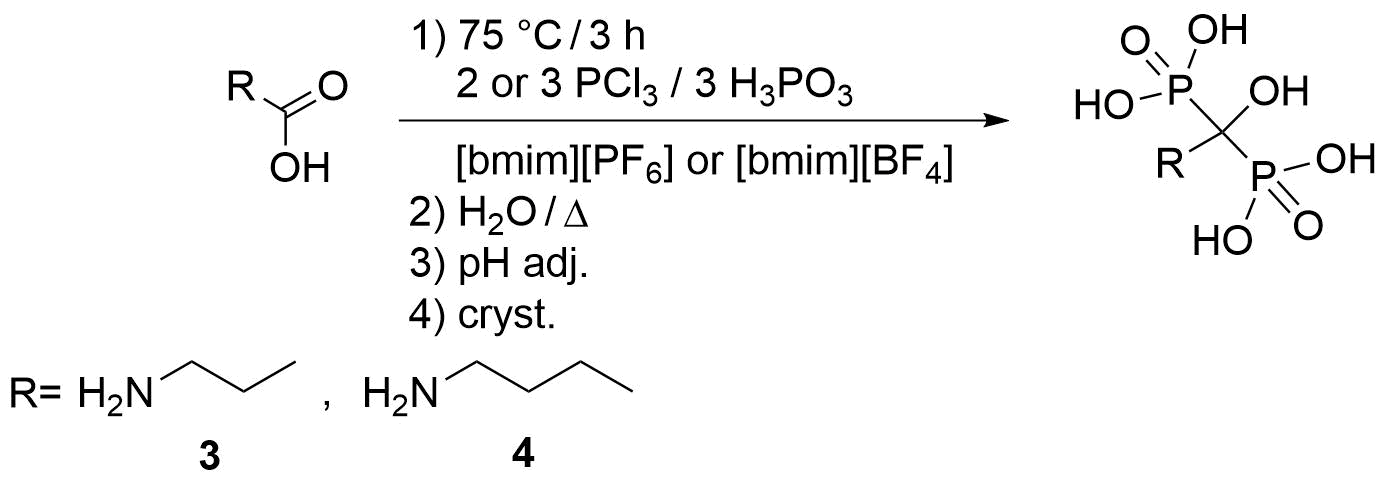
Although the synthesis of dronic acid derivatives is widely discussed in literature, it can be considered a kind of “black box”. Their published data are often confusing, misleading and unreliable. During the most common preparation, the corresponding carboxylic acid or its derivatives (acid chloride, anhydride or ester) were reacted with phosphorus trichloride and/or phosphorous acid (or in few instances, with phosphoric acid, phosphoryl chloride, phosphorus pentachloride or phosphorus trioxide) [3,S7]. They were prepared in many solvents, but the optimum circumstances and the molar ratios of the P-reagents were not explored. The role of the reagents and the reaction mechanism were not clarified. During our research, including my own work, our aim is to clarify the contradictions, explore the optimal conditions of the reactions, choose the appropriate P‑reagents and determine their optimum ratios. Overall, we wish to develop an efficient, high yield providing and more environmentally friendly synthesis of the pharmaceutically important bisphosphonic acids. We wish to realize the syntheses of dronic acid derivatives in environmentally friendly manner, using for example sulfolane or ionic liquids (ILs) instead of the widely used not robust methanesulfonic acid (MSA).
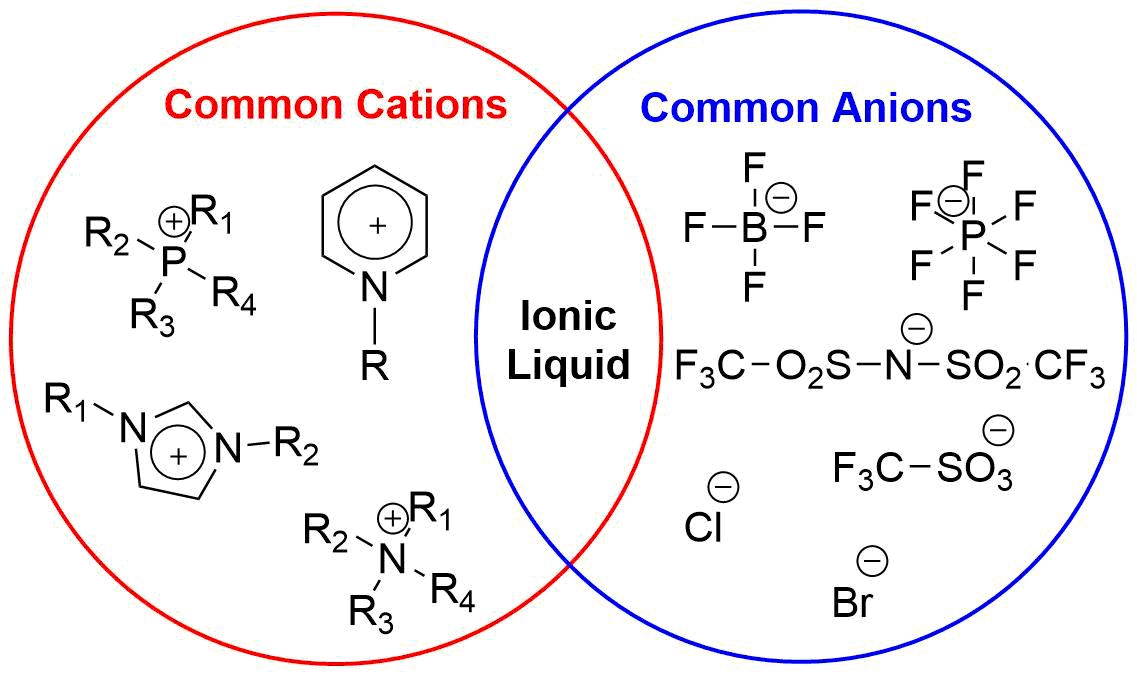
Fig. 3: The most commonly used ionic liquids in the organic syntheses
Nowadays, the use of ILs is spreading in synthetic organic chemistry, as they are considered ”green” solvents, because of their low vapor pressure, high thermal stability, and because they can be recycled or reused. In many cases, the ILs were only used as additives or catalysts. We started investigating the synthesis of α-hydroxymethylenebisphosphonic acid derivatives in ILs as first. Choosing the most appropriate IL and finding its optimum amount is an essential component of the research.
The role of the solvents and reagents has not been clarified (Fig. 4); their evaluation is necessary in the optimization of the syntheses, in making them more environmentally friendly, and in the exploration of the reaction mechanism.

Fig. 4: What can happen in the flask? [S10]
Methodology
All compounds were synthesized using up-to-date methods of synthetic organic chemistry. During the experiments, the corresponding carboxylic acid was reacted with phosphorus trichloride and phosphorous acid in different molar ratios in MSA, sulfolane or IL, at the optimum temperature. Designing the experiments required a high degree of circumspection. During the study, we have experienced surprising and interesting results that guided us in new directions.
We applied nuclear
magnetic resonance spectroscopy
(1H, 13C and 31P NMR) for the verification of
the chemical structure of the synthesized compounds.
The phosphonic groups of bisphosphonic acids provide strong ionic character
and increased polarity to the compounds, which makes the analysis of the
dronic acid derivatives complicate.
The dronate content of the synthesized samples was determined by potentiometric acid-base titration, which is a simple, accurate, fast and the most reliable method for testing of these compounds. The active ingredient content of our samples was determined by me, in Dorog by Richter Gedeon Plc. I had to work out the syntheses of the standard samples in analytical purity, which is essential to the evaluation of titration curves.
The crystalline water content of the compounds synthesized
was determined by
thermogravimetric analysis.
Based on the molar ratios experiments, we proposed reaction mechanisms for the
formation of bisphosphonic acids in different solvents, which concepts were
supported by
quantum chemistry calculations
in higher level.
Results
Before our research, the synthesis of bisphosphonic acid derivatives was considered a “black box”. Contrary to the literature, in the case of many dronic acids (etidronate (1) [S1], fenidronate (2) [S2], pamidronate (3) [6], alendronate (4) [7], ibandronate (5) [7], zoledronate (6) [8] and risedronate (7) [8]), it was demonstrated that the real P reagent is phosphorus trichloride, and its optimum amount is three equivalents in MSA. Phosphorous acid does not participate in the reaction because of its low nucleophilicity, thus it is only unnecessary ballast. During the preparation of benzidronate (8) [S3] and 3-phenylpropidronate (9) [S4], unexpected results were observed in MSA. When only phosphorous trichloride was the P-reactant, no dronic acid was formed. There was need to use both P-reagents (PCl3 and H3PO3) at the same time. As an explanation in previous cases (1-7), the intermediate Cl2P-O-SO2Me (10) may be the active P-reagent, while in the case of benzidronate (8) and 3-phenylpropidronate the adducts (HO)2P-O-PCl2 (11) or (HO)2P-O-PCl-O-P(OH)2 (12) were assumed as nucleophiles, and they react with the carboxylic acid chloride (13) or mixed anhydride (14) formed in the reaction mixture (Fig. 5).
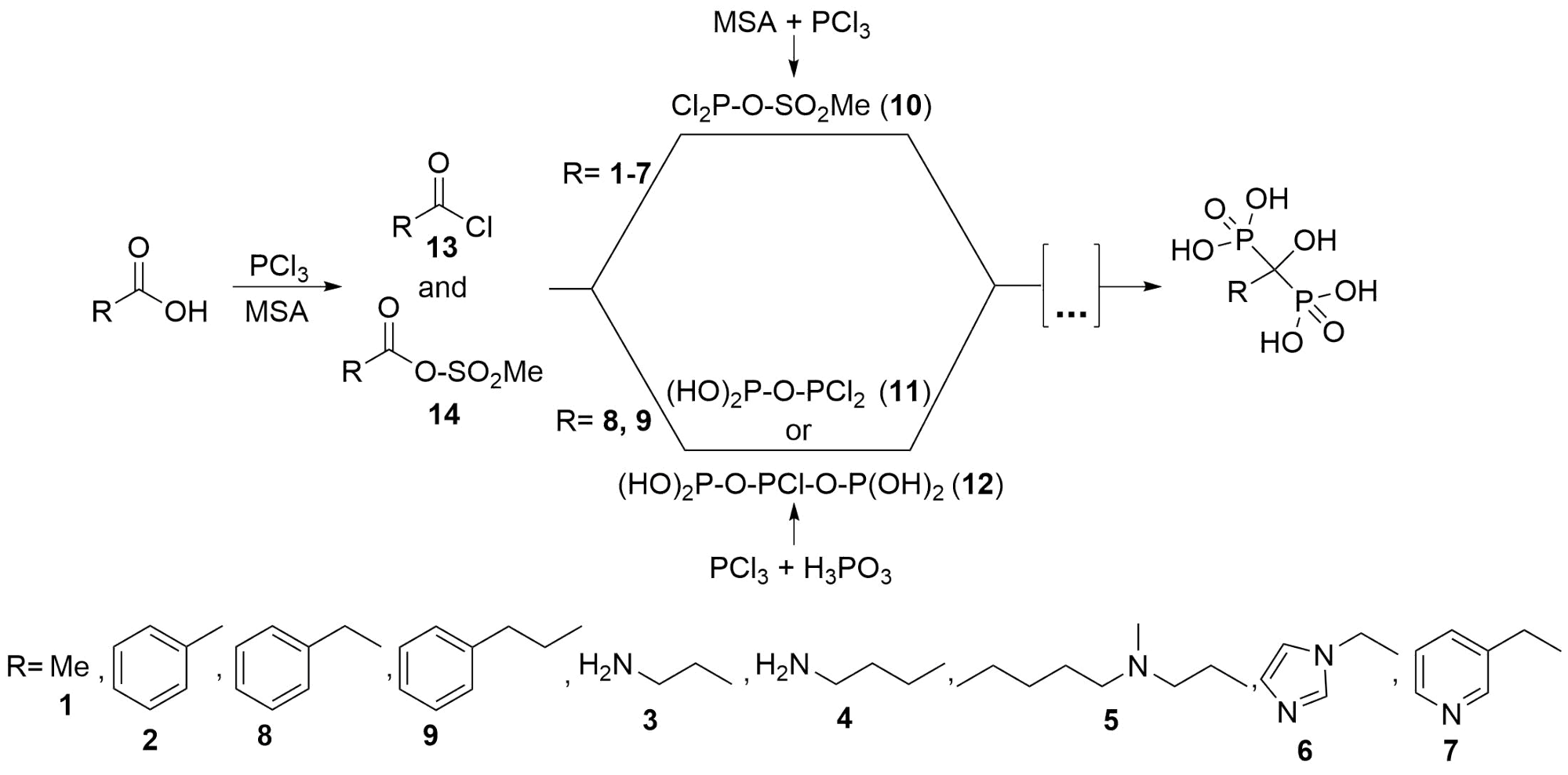
Fig. 5: Possible syntheses in MSA [S1-S4,6-8]
The other frequently used solvent in the synthesis of dronic acid derivatives is sulfolane. The preparation of pamidronic acid (3) [6] and alendronate (4) [S9] was studied in this solvent. Surprisingly, when only phosphorous trichloride was the P-reactants, no dronic acid was formed. Also, both P-reagents (PCl3 and H3PO3) had to be applied simultaneously. We proved that the solvents have an important role, as determine which P-reactants participate in the formation of bisphosphonates, thus they also have a significant effect on the mechanism of the reactions. Logically, the (HO)2P-O-PCl2 (11) and/or (HO)2P-O-PCl-O-P(OH)2 (12) species may also be the real nucleophiles that react with the starting carboxylic acid or carboxylic acid chloride, which may form “in situ” during the reaction.
The synthesis of α-hydroxymethylenebisphosphonic acid derivatives has also been investigated in ILs by us as first (Fig. 6) [S5,S8,S9]. It was necessary to measure in both phosphorus trichloride and phosphorous acid as the P-reagents as in sulfolane.
Fig. 6: Synthesis of pamidronic acid (3) and alendronate (4) in the presence of ILs
We found that there was no need for using the ILs as a solvent, only in smaller, catalytic amounts. Their optimal amounts fell in a range of 0.3-0.6 equivalents. Increasing the amount of ILs (using as a solvent), the yields decreased. They have a negative effect to the reactions in larger quantity. The ILs have a really significant impact on the syntheses of alendronate (4) (Fig. 7).
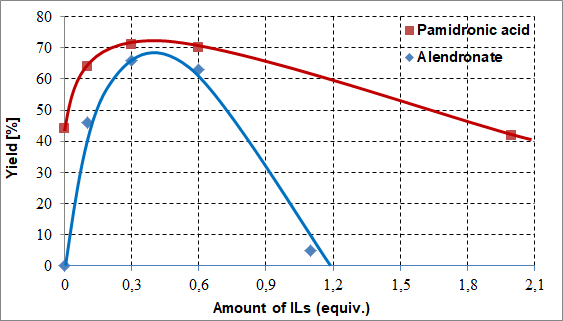
Fig. 7: The effect of the amount of the proper ILs to the yield of pamidronic acid (3) ([bmim][BF4]) and alendronate (4) ([bmim][PF6]) [S5,S8,S9]
It was assumed that the presence of an IL may increase the electrophilic character of the carbonyl group of the carboxylic acid derivatives, thus helping their reaction with the activated P-reactants (Fig. 8).
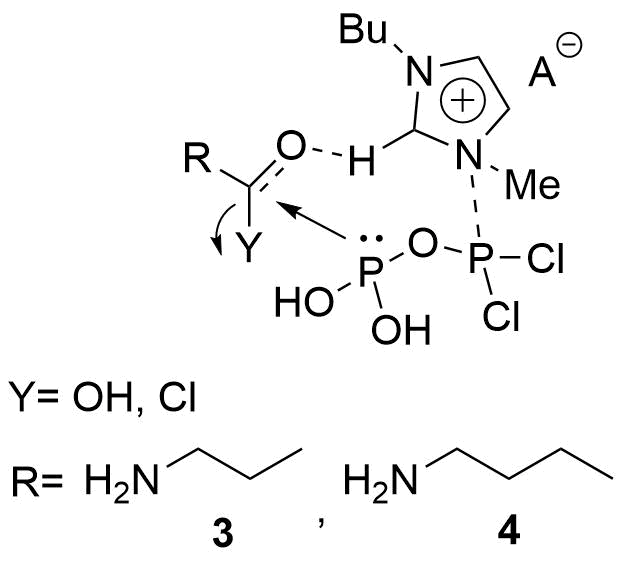
Fig. 8: The possibly catalytic effect of the IL additive
Expected impact and further research
In the future, we wish to carry out the syntheses of further important α-hydroxymethylenebisphosphonic acids in the presence of ILs, which can be a significant step forward in making the preparation of these compounds. The exploration of the real reaction mechanism and the explanation of the positive effects of ILs on the reactions are a challenging project, to which we wish to contribute. We wish to underpin our ideas with quantum chemical calculations. We also share our findings on the proper use of ILs.
Publications, references, links
Publications:
[S1] Kovács, R.; Nagy, D. I.; Grün, A.; Balogh, Gy. T.; Garadnay, S.; Greiner, I.; Keglevich, G. Lett. Drug. Des. Discov., 2013, 10, 733-737.
[S2] Kovács, R.; Nagy, D. I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Lett. Org. Chem., 2014, 11, 368-373.
[S3] Kovács, R.; Nagy, D. I.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Lett. Drug. Des. Discov., 2015, 12, 78-84.
[S4] Grün, A.; Nagy, D. I.; Németh, O.; Mucsi, Z.; Garadnay, S.; Greiner, I.; Keglevich, G. Curr. Org. Chem., 2016, 20, 1745-1752.
[S5] Grün, A.; Nagy, D. I.; Garadnay, S.; Greiner, I.; Keglevich, G. Lett. Drug. Des. Discov., 2016, 13, 475-478.
[S6] Nagy, D. I., Grün, A., Garadnay, S., Greiner, I., Keglevich, G. Phosphorus, Sulfur Silicon Relat. Elem., 2016, 191, 1619-1620.
[S7] Nagy, D. I., Grün, A., Garadnay, S., Greiner, I., Keglevich, G. Molecules., 2016, 21, 1046-1064.
[S8] Kiss, N. Z., Nagy, D. I., Keglevich, G. in Advances in chemistry research; Taylor J. C., Ed.; Nova Science Publishers: New York, 2017; Vol. 37, pp. 121-140.
[S9] Nagy, D. I., Grün, A., Garadnay, S., Greiner, I., Keglevich, G. Heteroatom Chem., 2017, 28, Article No.: 21370.
[S10] Nagy, D. I., Grün, A., Greiner, I., Keglevich, G. Curr. Org. Chem., 2017, 21, 1567-1578.
Links:
References:
[1] Fleisch, H.; Russell, R. G. G.; Straumann, F. Nature, 1966, 212, 901-903.
[2] Breuer, E. In Analogue-based drug discovery, 2006, 371-384.
[3] Hudson, H. R.; Wardle, N. J.; Bligh, S. W. A.; Greiner, I.; Grün, A.; Keglevich, G. Mini Rev. Med. Chem., 2012, 12, 313-325.
[4] Russell, R. G. G. Bone, 2011, 49, 2-19.
[5] Catterall, J. B.; Cawston, T. E. Arthritis Res. Ther., 2002, 5, 12-24.
[6] Kovács, R.; Grün, A.; Németh, O.; Garadnay, S.; Greiner, I.; Keglevich, G. Heteroatom Chem., 2014, 25, 186–193.
[7] Kovács, R.; Grün, A.; Garadnay, S.; Greiner, I.; Keglevich, G. Curr. Org. Synth., 2013, 10, 640-644.
[8] Grün, A.; Aradi, K; Garadnay, S.; Greiner, I.; Keglevich, G. Tetrahedron Lett., 2011, 52, 2744-2746.