|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Dr. György Keglevich
Synthesis of organophosphorus compounds in a continuous flow microwave reactor
Introducing the research area
During our research work, we have developed a continuous flow microwave (MW) system for the scaled-up preparation of organophosphorus compounds.
Brief introduction of the research place
Our work was conducted at the Department of Organic Chemistry and Technology in the Green Chemical and Organophosphorus Research Group. One main purpose of our group is to investigate the environmentally friendly synthesis of organophosphorus compounds.
History and context of the research
In the past few years, flow reactors have started to find place in modern laboratories [1]. Although the limitations of flow reactions, such as the problem of heterogeneity or high viscosity mean significant drawbacks, the more efficient control of reaction conditions, the easier real time analysis and the safer handling of hazardous reactions slowly convince the academic and industrial community. Moreover, flow reactions may be significantly better in selectivity and yields as compared to batch approaches. In microwave (MW) chemistry, the flow chemistry has solved a very serious issue [2]. Due to the limited geometry of the MW devices, the scale-up of MW-assisted reactions is a real challenge. By the use of continuous flow MW reactors, this problem can be eliminated (Figure 1).
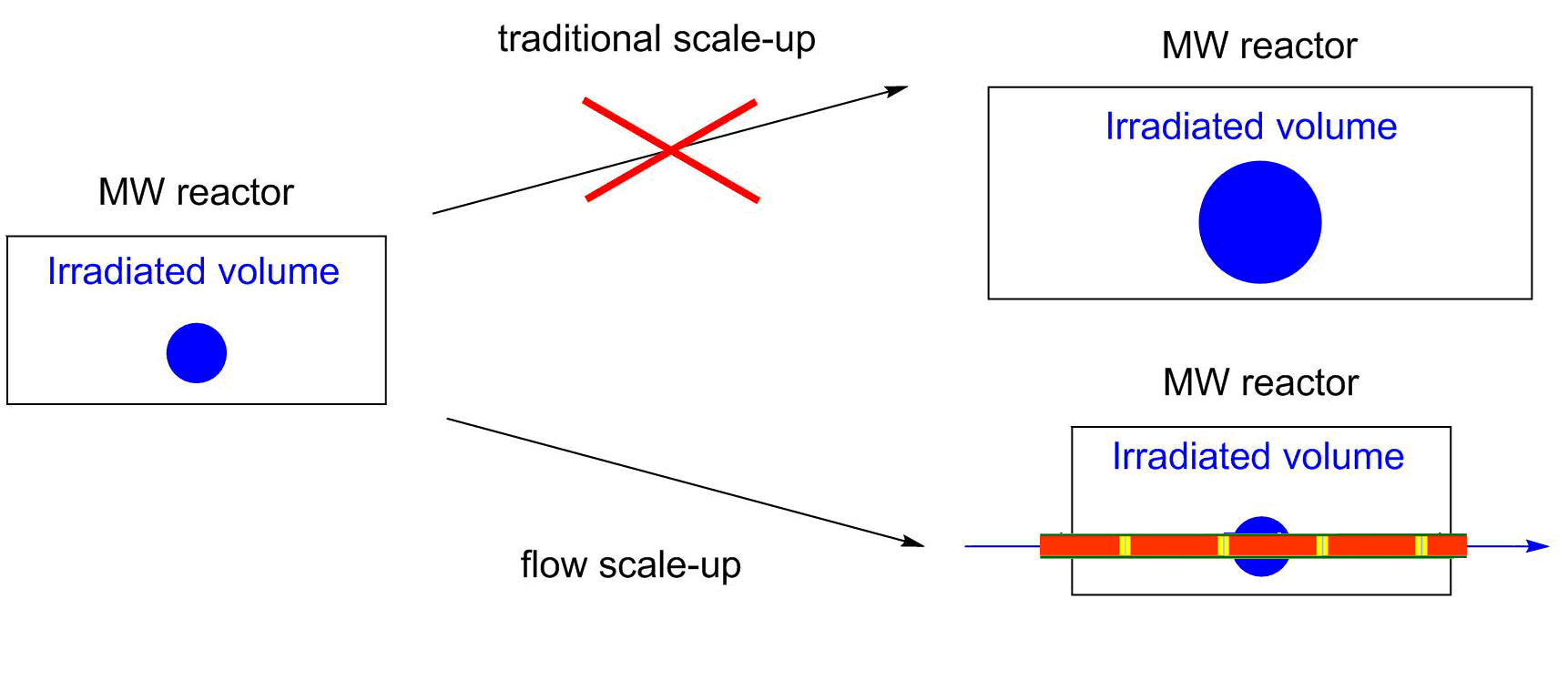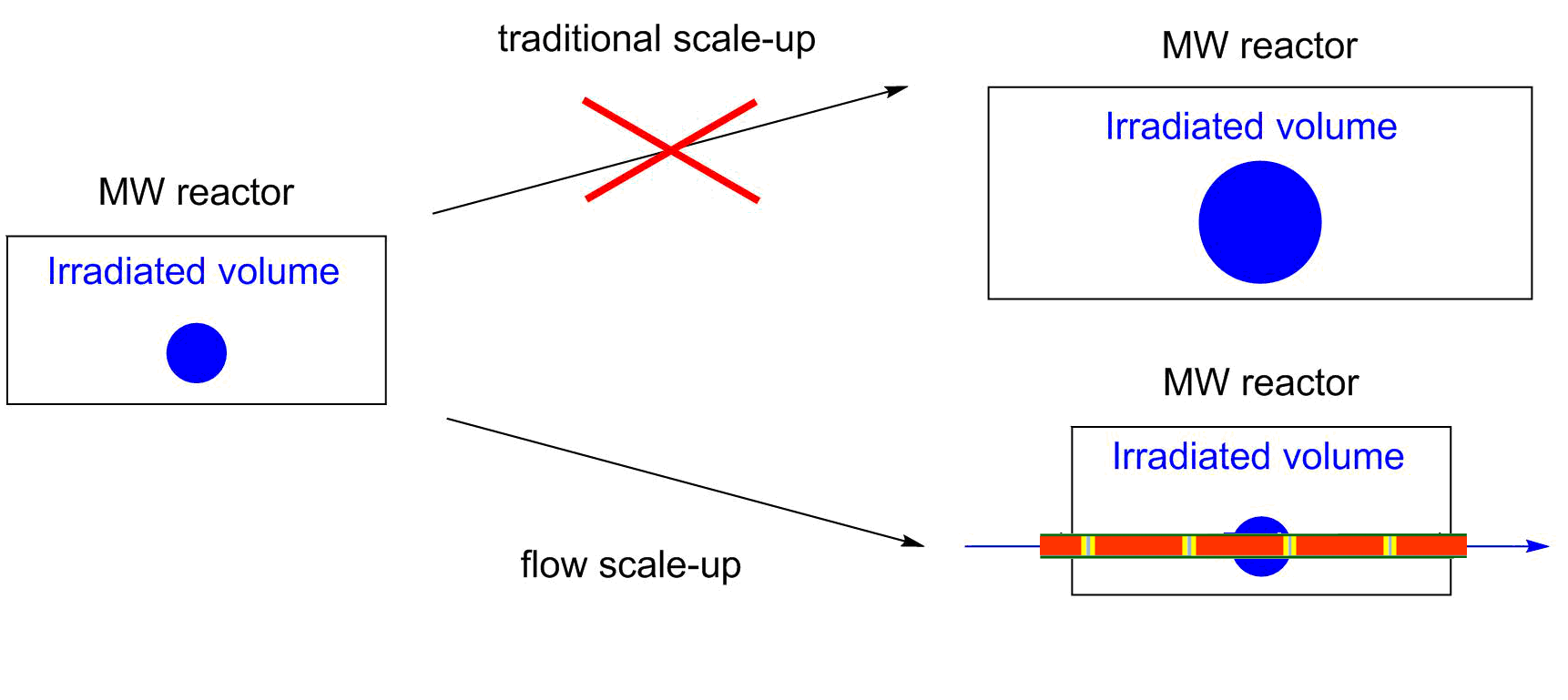
Figure 1: Scaling up of MW-assisted reactions
During the last decade, several applications of continuous flow MW reactors were reported [3], however, due to the many non-professional MW reactors; the reproduction of these reactions is often problematic. In order to monitor and control the most important parameters (such as temperature and pressure), the application of professional devices is required.
The research goals, open questions
The purpose of our research was to develop a continuous flow MW system for the synthesis of organophosphorus derivatives. In our model reactions, the preparation of dialkyl H-phosphonates (dialkyl phosphites) and α-aminophosphonates was investigated (Scheme 1).
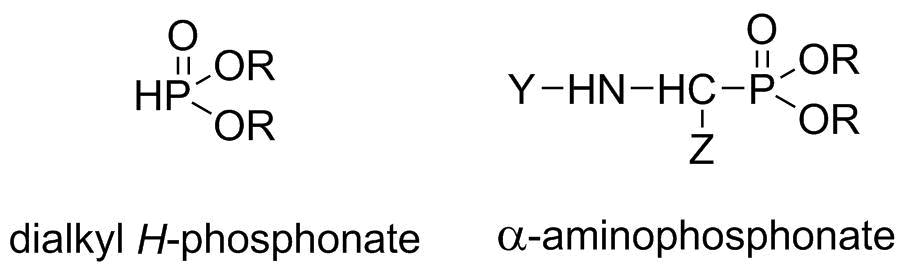
Scheme 1: Structures of the target compounds
Dialkyl phosphites are important starting materials in the synthesis of bisphosphonates, α-hydroxyphosphonates and α-aminophosphonates [4]. One possible preparation of dialkyl phosphites is the alcoholysis. In this environmentally friendly procedure, the reaction of a dialkyl phosphite and an alcohol takes place; the two alkoxy groups of the starting material consecutively change to two different ones. Both product types that can be prepared are valuable compounds.

Scheme 2: Synthesis of dialkyl phosphites by alcoholysis
α-Aminophosphonates are the structural analogues of α-amino acids. In their backbone, the carboxyl-function (-C(O)OH) is replaced by a phosphonate moiety (-P(O)(OR)2) [5]. Due to this similarity, they are of potential biological activity, such as antibiotic, antiviral or anticancer agents [S1]. Beside their medicinal application, they play an important role in agriculture as herbicides (e.g. glyphosate) and fungicides. The two most important synthetic routes towards α-aminophosphonates are the aza-Pudovik reaction (A) and the Kabachnik-Fields condensation (B) (Scheme 3) [6, S2].
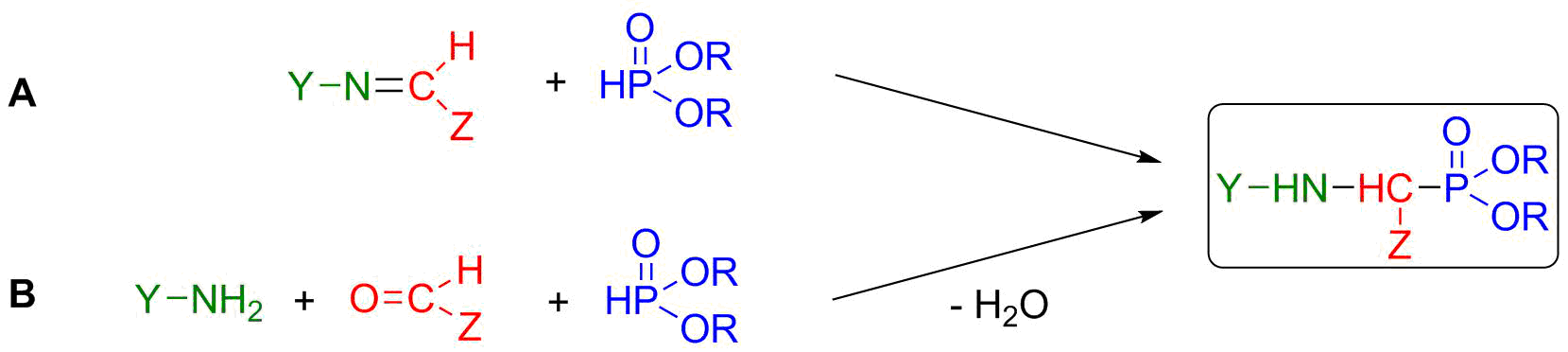
Scheme 3: Synthesis of α-aminophosphonates by aza-Pudovik (A) and Kabachnik-Fields (B) reaction
Methods
Our continuous flow system is based on a commercially available CEM Discover batch MW reactor and a CEM continuous flow cell (Figure 2) [S3]. In the flow cell, the mixture gets to the bottom of the vessel (yellow arrows), and after saturating, leaves on the top hole (red arrows). The MW irradiation, which is provided by the magnetron of the reactor, takes place when the mixture is present in the cell.
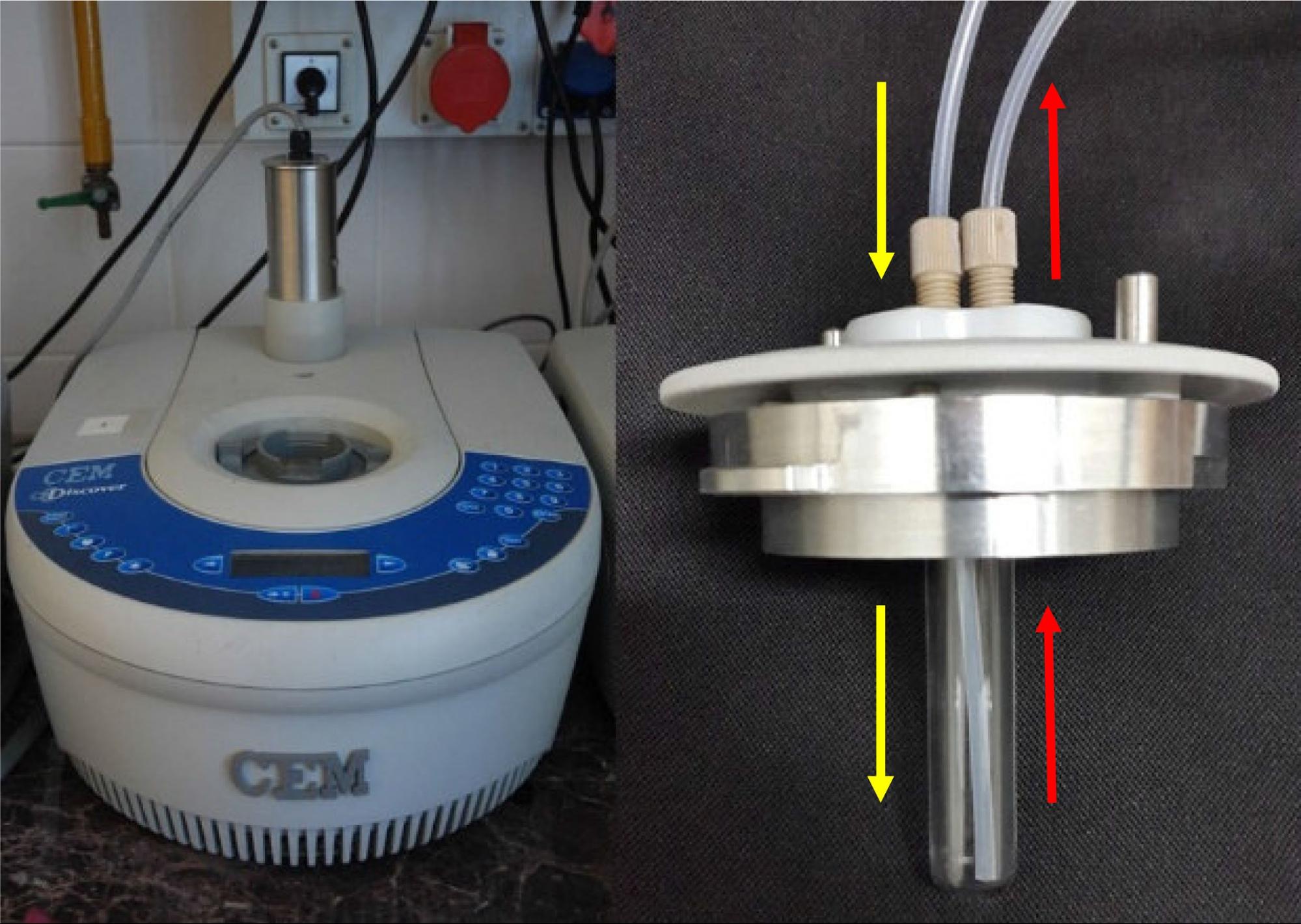
Figure 2: The MW reactor (l) and the flow cell (r)
Two of our model reactions, the alcoholysis and the aza-Pudovik reaction, comprise two-two starting materials so the selectivity is not an issue. None of these reactions takes place at room temperature, so the reagents can be mixed and being fed together. These procedures require only one pump in the system. In contrast, in case of the Kabachnik-Fields reaction, the aldehyde reacts with both the dialkyl phosphite and the amine even at room temperature causing by-products. To avoid the by-product formation, the aldehyde must be fed separately from the two other starting materials. This requires at least two pump. In this system, the two flow streams are mixed just before entering the reactor. The schematic drawings of the two systems developed can be seen on Figure 3.
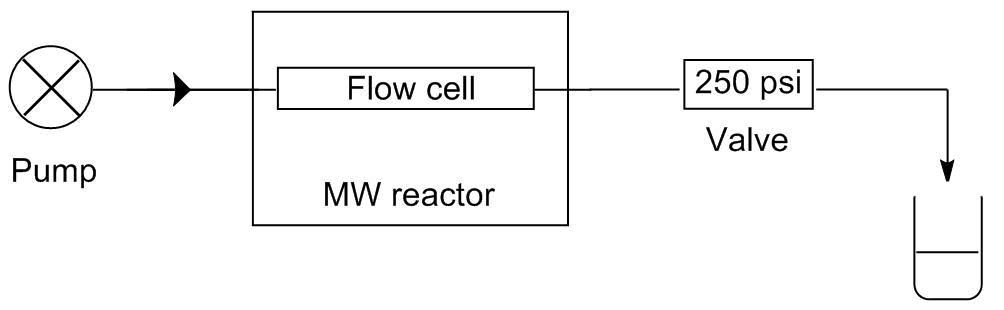
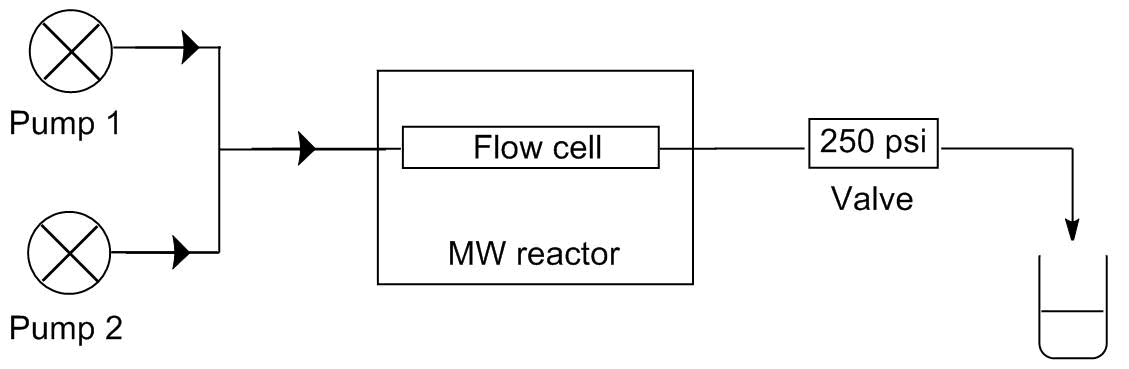
Figure 3: Schematic drawings of the one-pump (l) and the two-pump (r) flow systems
The reagents were fed into the reactor by HPLC pumps. The mixture leaving the reactor was passed through a cooler and a back pressure regulator (17 bar). In the end of the system the product reached the product collector at room temperature and atmospheric pressure. The leaving mixture was continuously monitored by gas and liquid chromatography. The detailed pictures of the MW systems developed can be seen on Figure 4 and 5.
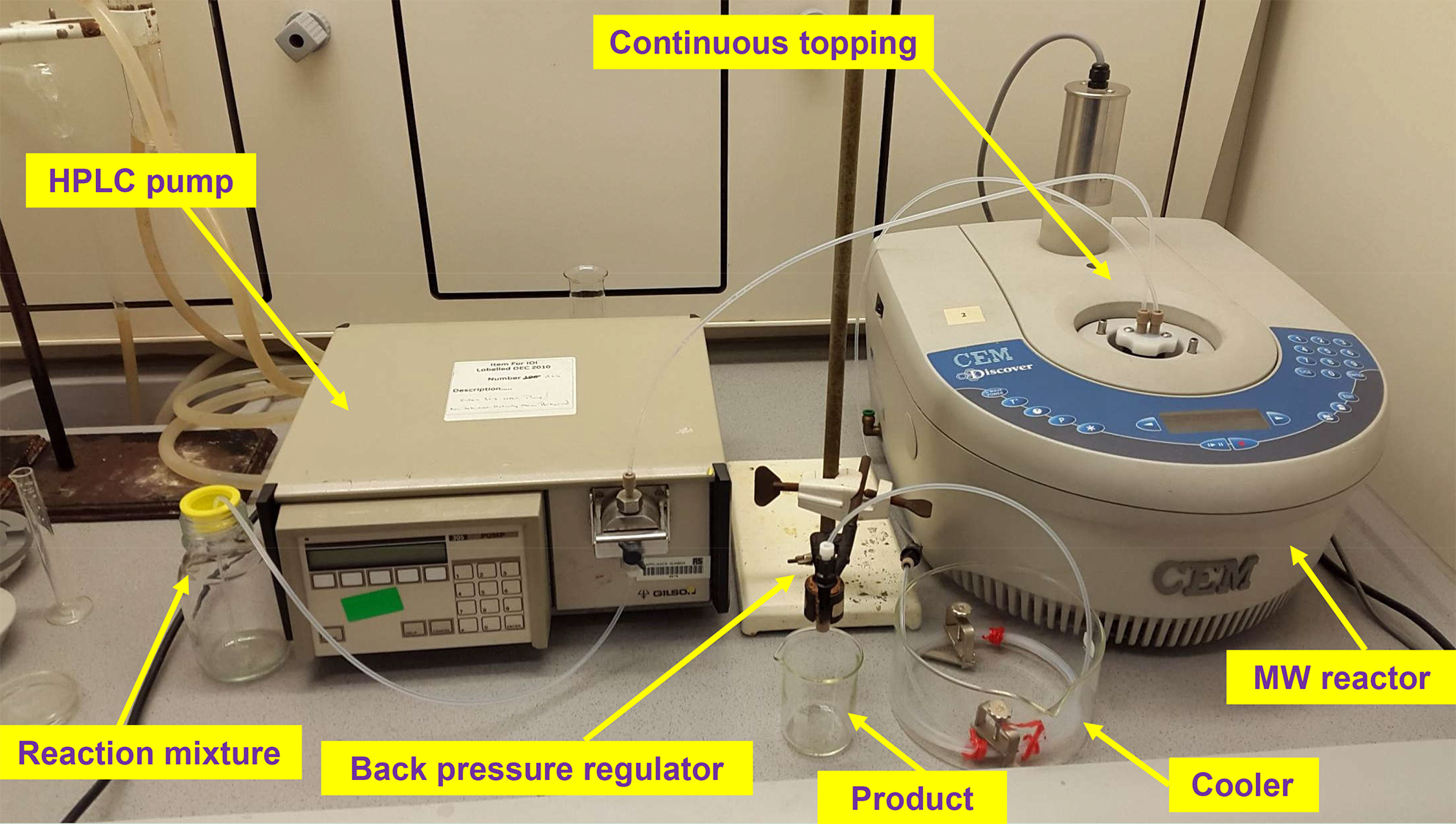
Figure 4: Picture of the one-pump continuous flow system
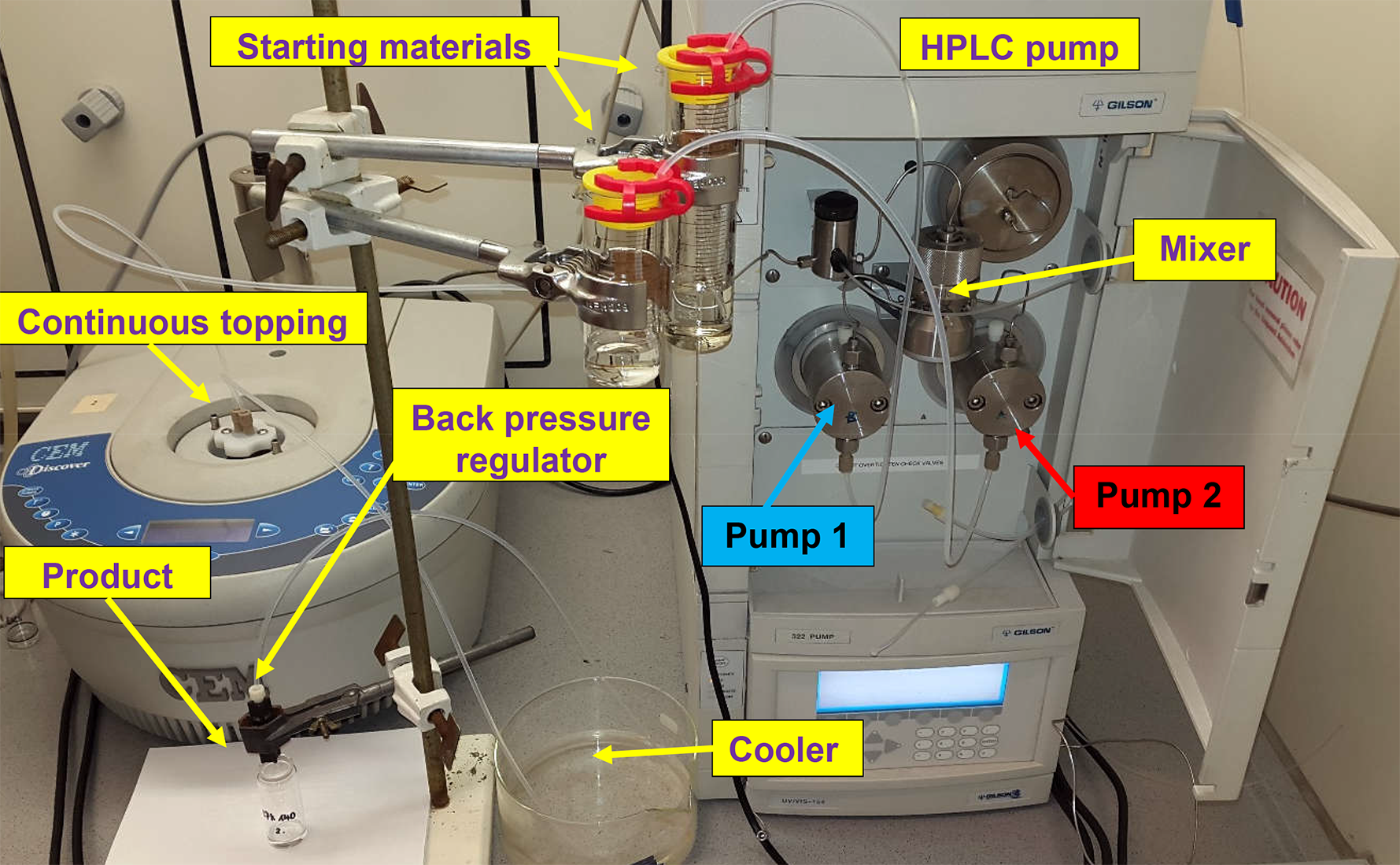
Figure 5: Picture of the two-pump continuous flow system
In order to optimize the reactions, the retention time (or reaction time) can be modified by changing the flow rate on the HPLC pumps. The temperature is monitored and controlled by the infrared (IR) sensor of the MW reactor. Due to the back pressure regulator, the pressure in the system is constant 17 bar, what makes possible the application of volatile solvents or reagents above their atmospheric boiling point.
Results
In the first step, the system developed was tested in a simple reaction: in the direct esterification of benzoic acid [S3]. The purpose of these model experiments was to gather information about the system and test the applicability range. The first reactions were carried out in batch mode, and the most suitable conditions were transferred to the flow system, where the parameters were optimized further. The system proved to be stable and efficient in a wide range of residence time (5-60 min) and reaction temperature (25-200 °C). After that, organophosphorus derivatives were prepared.
The flow alcoholysis of dialkyl phosphites was carried out based on our previous experiences with this reaction in a batch MW reactor [S4-S6]. In the flow approaches, similarly to the batch experiments, by changing the reaction conditions, the alcoholysis of dialkyl phosphites could be fine-tuned towards the desired products [S7]. At lower temperatures, the mixed derivatives formed as the main products, while at higher temperatures, the fully transesterified phosphites predominated (Scheme 4). The main advantages of the continuous flow operation are the shorter reaction time and the larger productivity.
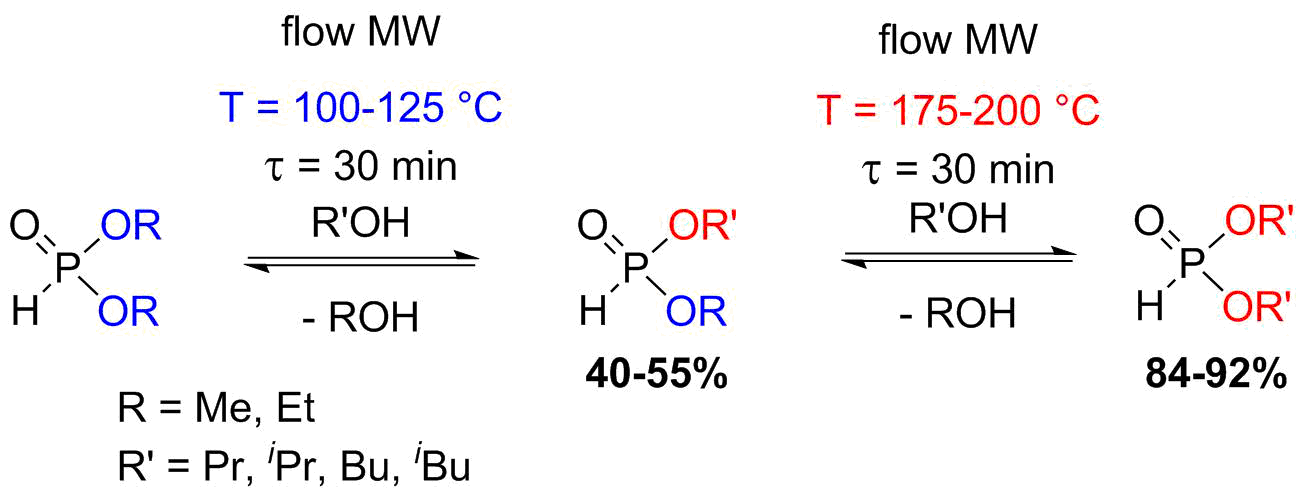
Scheme 4: Flow alcoholysis of dialkyl phosphites by aliphatic alcohols
During our previous research, it was revealed that the most efficient and environmentally friendly way of aza-Pudovik reactions is the solvent- and catalyst-free MW-assisted accomplishment [S8]. However, in a flow system, one of the most important criteria is to avoid heterogeneity. Therefore, we had to make this reaction flow-compatible. Several “green” solvents were tried out and ethanol proved to be the best. The aza-Pudovik reactions were optimized in the flow system, and all target α-aminophosphonates could be prepared selectively with full conversion (Scheme 5) [S9].
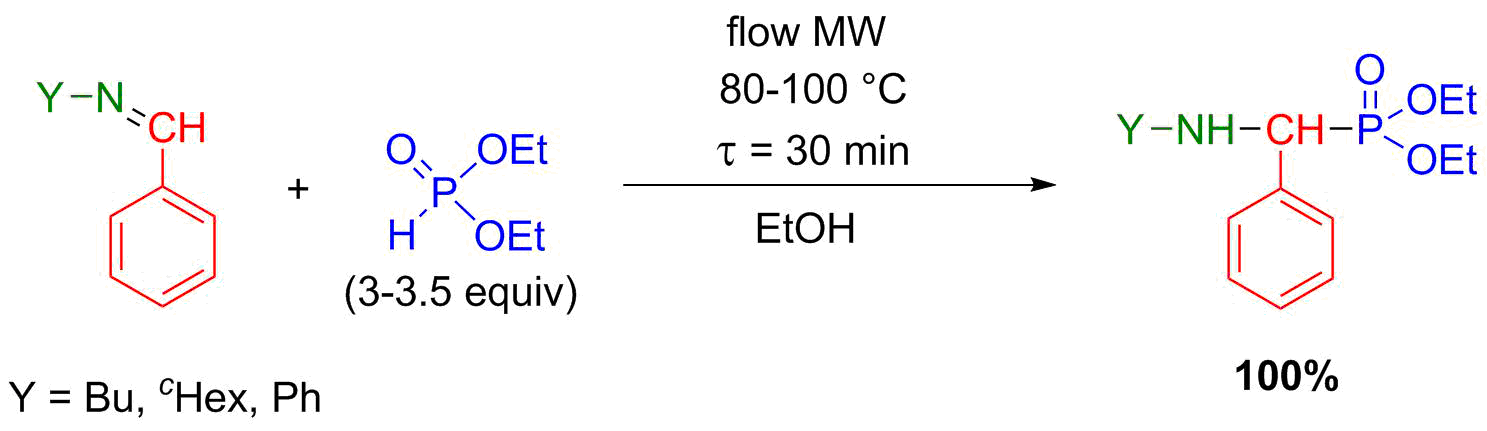
Scheme 5: Flow synthesis of α-aminophosphonates by aza-Pudovik reaction
In the synthesis of α-aminophosphonates by the Kabachnik-Fields reaction, similar considerations are required as in the aza-Pudovik reactions. The three-component condensation is the most efficient and environmentally friendly if it is carried out without any catalyst or solvent in a MW reactor [S10, S11]. Otherwise, if the scale-up is necessary, flow-compatible conditions are required. The Kabachnik-Fields condensations (similarly to the aza-Pudovik reactions) were carried out in ethanol. Multicomponent reactions are not so clear cut in flow systems [7]. In our experiments, starting from aliphatic amines, the reactions were indeed not selective (more or less α-hydroxyphosphonates also formed), whereas when applying aromatic amines as starting materials, the target α-aminophosphonates were obtained selectively (Scheme 6) [S9].
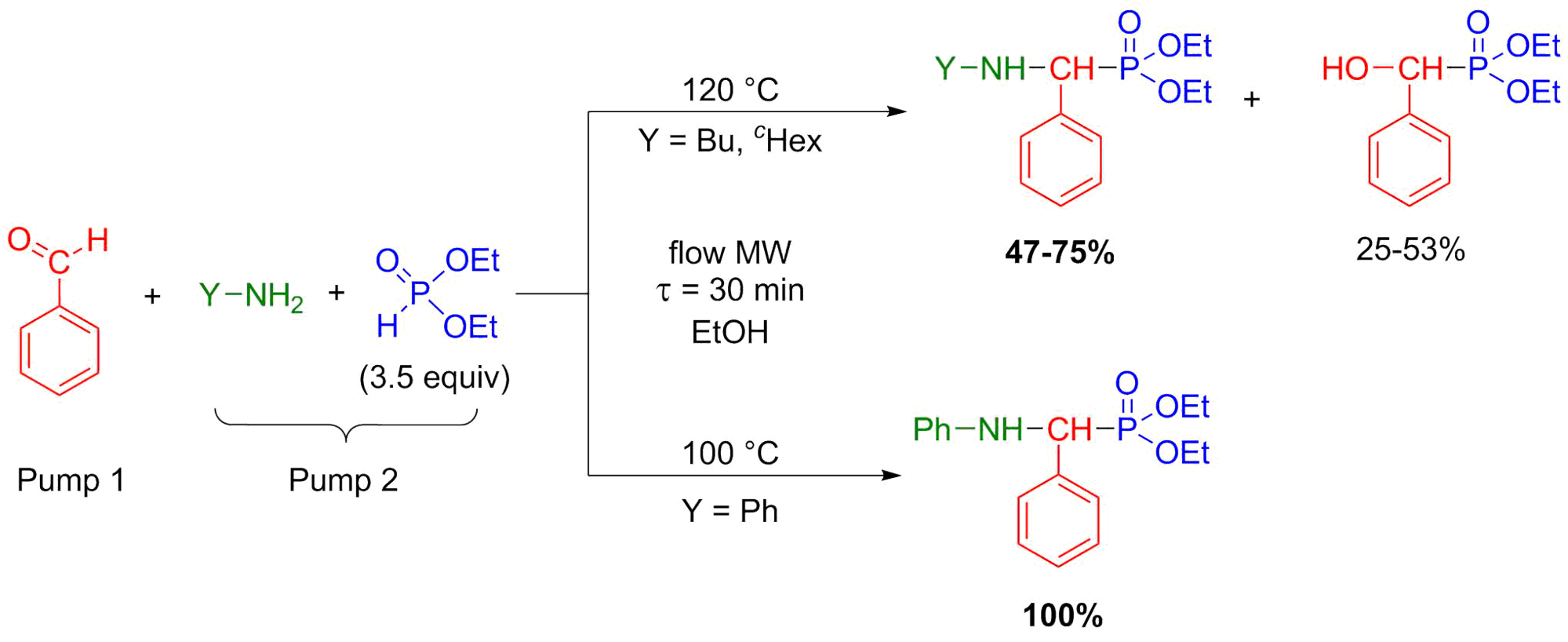
Scheme 6: Flow synthesis of α-aminophosphonates by Kabachnik-Fields reaction
Expected impact and further research
The continuous flow MW reactors have been evolving steadily. We may assume that the presentation of the flow systems designed and the reactions carried out by us can significantly contribute to this evolution.
Our one-pump and two-pump systems allow efficient implementations of the simpler two-component and multicomponent reactions in a continuous flow manner. As a result of our research, the synthesis of important organophosphorus starting materials (dialkyl phosphites) and potentially bioactive derivatives (α-aminophosphonates) was accomplished on a larger scale.
Our plans for the future include the synthesis of heterocyclic organophosphorus compounds by flow multicomponent reactions, where the flow chemistry can play an important role in obtaining the target products more selectively.
Publications, references, links
List of corresponding own publications:
[S1] Tajti, Á.; Keglevich, G. The importance of organophosphorus compounds as biologically active agents, In: Keglevich, G. (ed.) Organophosphorus Chemistry, Berlin: Walter de Gruyter GmbH, 2018. Ch. 3, pp. 53‒65.
Doi: 10.1515/9783110535839-003
[S2] Bálint, E.; Tripolszky, A.; Tajti, Á. Synthesis of α-aminophosphonates
by the Kabachnik–Fields reaction and by the Pudovik reaction, In: Keglevich,
G. (ed.) Organophosphorus Chemistry, Berlin: Walter de Gruyter GmbH,
2018. Ch. 6, pp. 108‒147.
Doi:
10.1515/9783110535839-006
[S3] Tajti, Á.; Tóth, N.; Bálint, E.; Keglevich, G. Esterification of
benzoic acid in a continuous flow microwave reactor, J. Flow Chem.
2018, 8, 11‒19. IF (2017): 1.658
Doi:
10.1007/s41981-018-0001-x
[S4] Bálint, E.; Tajti, Á.; Drahos, L.; Ilia, G.; Keglevich, G., Alcoholysis
of dialkyl phosphites under microwave conditions, Curr. Org. Chem.
2013, 17, 555‒562. IF:2.537
Doi:
10.2174/1385272811317050010
[S5] Keglevich, G.; Bálint, E.; Tajti, Á.; Mátravölgyi, B.; Balogh, G. T.;
Bálint, M.; Ilia, G.; Microwave-assisted alcoholysis of dialkyl phosphites by
ethylene glycol and ethanolamine, Pure Appl. Chem. 2014, 86,
1723‒1728. IF:2.615
Doi:
10.1515/pac-2014-0601
[S6] Bálint, E.; Tajti, Á.; Drahos, L.; Ilia, G.; Keglevich, G., Alcoholysis
of dialkyl phosphites under microwave conditions, Curr. Org. Chem.
2013, 17, 555‒562. IF:2.537
Doi:
10.2174/1385272811317050010
[S7] Bálint, E.; Tajti, Á.; Tóth, N.; Keglevich, G. Continuous flow alcoholysis of dialkyl H-phosphonates with aliphatic alcohols, Molecules 2018, accepted for publication, IF(2017): 3.098
[S8] Bálint, E.; Tajti, Á.; Ádám, A.; Csontos, I.; Karaghiosoff, K.; Czugler, M.; Ábrányi-Balogh, P.; Keglevich, G. The synthesis of α-aryl-α-aminophosphonates and α-aryl-α-aminophosphine oxides by the microwave-assisted Pudovik reaction, Beilstein J. Org. Chem. 2017, 13, 76‒86. IF: 2.330
Doi: 10.3762/bjoc.13.10
[S9] Bálint, E.; Tajti, Á.; Ladányi-Pára, K.; Tóth, N.; Keglevich, G., in preparation.
[S10] Tajti, Á.; Bálint, E.; Keglevich, G. Synthesis of Ethyl Octyl α-Aminophosphonate
Derivatives, Curr. Org. Synth. 2016, 13, 638‒645.
IF:2.050
Doi:
10.2174/1570179413666151218202757
[S11] Bálint, E.; Tajti, Á.; Kalocsai, D.; Mátravölgyi, B.; Karaghiosoff, K.;
Czugler, M; Keglevich, G. Synthesis and utilization of optically active α-aminophosphonate
derivatives by Kabachnik-Fields reaction, Tetrahedron, 2017,
73, 5659‒5667. IF: 2.377
Doi:
10.1016/j.tet.2017.07.060
List of indirectly related own publications:
[S12] Bálint, E.; Fazekas, E.; Takács, J.; Tajti, Á.; Juranovič, A.; Kočevar, M.; Keglevich, G. Microwave-Assisted Synthesis of Organophosphorus Compounds, Phosphorus, Sulphur, Silicon 2012, 188, 48‒50. IF:0.827
Doi: 10.1080/10426507.2012.743544
[S13] Bálint E.; Tajti, Á.; Dzielak, A.; Hägele, G.; Keglevich, G. Microwave-assisted synthesis of amino-methylene-bisphosphine oxides and amino-methylene-bisphosphonates by a three-component condensation, Beilstein J. Org. Chem. 2016, 12, 1493‒1502. IF:2.300
Doi: 10.3762/bjoc.12.146
[S14] Bálint, E.; Tripolszky, A.; Ádám, A.; Tajti, Á.; Keglevich, G. Synthesis and utilization of α-aminophosphine oxides and related derivatives, Phosphorus, Sulfur, Silicon 2016, 191, 1539‒1540. IF:0.809
Doi: 10.1080/10426507.2016.1212860
[S15] Tajti, Á.; Tóth, R. E.; Kalocsai, D.; Keglevich, G.; Bálint, E. Formation of compounds with P–C–N moiety by microwave-assisted condensations, Phosphorus, Sulfur, Silicon 2016, 191, 1541‒1542. IF:0.809
Doi: 10.1080/10426507.2016.1212861
[S16] Amadeu, N.; Bálint, E.; Boenigk, W.; Tajti, Á.; Hägele, G.; Janiak, C.; Keglevich, G. NMR and symmetry in bisphosphonates R1R2N-CH[P(O)(OMe2)2], Phosphorus, Sulfur, Silicon 2017, 192, 643‒650. IF:0.674
Doi: 10.1080/10426507.2017.1295966
[S17] Bálint, E.; Tajti, Á.; Tripolszky, A.; Keglevich, G. Synthesis of platinum, palladium and rhodium complexes of α-aminophosphine ligands, Dalton Trans. 2018, 47, 4755‒4778. IF(2017): 4.099
Doi: 10.1039/C8DT00178B
[S18] Banerjee, B; Tajti, Á.; Keglevich, G. Ultrasound-assisted synthesis of
organophosphorus compounds, In: Keglevich, G. (ed.) Organophosphorus
Chemistry, Berlin: Walter de Gruyter GmbH, 2018. Ch. 13, pp. 248‒263.
Doi:
10.1515/9783110535839-013
Table of links:
Green Chemical and Organophosphorus Research Group
Dialkyl H-phosphonates (dialkyl phosphites)
List of references:
[1] Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. The Hitchhiker’s guide to flow chemistry, Chem. Rev. 2017, 117, 11796‒11893.
[2] Bálint, E.; Keglevich, G. The Spread of the Application of the Microwave Technique in Organic Synthesis. In Milestones in Microwave Chemistry, Keglevich, G., Ed.; Springer: Basel, Switzerland, 2016; pp. 1‒10.
[3] Estela, L.; Pouxb, M.; Benamaraa, N.; Polaerta, I. Continuous flow-microwave reactor: Where are we?, Chem. Eng. Process. 2016, 113, 56‒64.
[4] Troev, K. D. Chemistry and Application of H-Phosphonates, Elsevier: Amsterdam, The Netherlands, 2006.
[5] Kukhar, V. P.; Hudson, H. R. Aminophosphinic and Aminophosphinic Acids: Chemistry and Biological Activity, John Wiley & Sons Ltd, Chichester, 2000.
[6] Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use, Molecules 2012, 17, 12821‒12835.
[7] Mileghem, S. V.; Veryser, C.; de Borggraeve, W. M. Flow-Assisted Synthesis of Heterocycles via Multicomponent Reactions. In Flow Chemistry for the Synthesis of Heterocycles, Sharma U., Van der Eycken E., Ed.; Springer: Cham, Switzerland, 2018; pp. 133‒159.
