|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Physical Chemistry and Materials Science
Supervisor: Dr. Pukánszky Béla
Application of Natural Antioxidants as Processing Stabilizers for Polyethylene
Introducing the research area
Polyethylene (PE) [L1] is among the common plastics, with properties tunable over a broad range by the modification of its molecular weight, structure and distribution, which makes it an important material of packaging, construction and automobile industry. However, these properties may alter by degradation processes during processing, that can be bypassed by the application of stabilizers, most commonly synthetic phenolic products. The goal of our research is to examine, whether antioxidants with natural origin, primarily flavonoids [L2] are able to act as processing stabilizer of PE.
Brief introduction of the research place
The Laboratory of Plastic and Rubber Technology [L3] at the Department of Physical Chemistry and Materials Science in BME and the Polymer Physics Research Group [L4] at the Institute of Materials and Environmental Chemistry in MTA TTK have been carrying out a joint research on the processes occurred during the processing of polyolefins for decades. I joined this collaboration during my BSc studies.
History and context of the research
High temperature, shearing rate and the presence of oxygen lead to thermooxidative, autocatalytic, radical reactions during the processing of polyolefins [1], which alter the structure of polymer chains. These reactions can lead to random chain scissions, or to the formation of long chain branches, depending on the fine structure of the material and the actual values of environmental parameters [2]. The two routes affect the molecular weight of the polymer oppositely, but both phenomenon lead to difficulties during processing and fragile products.
Hindered phenolic substances are generally applied as primary stabilizers by the industrial practice. These substances are able to donate a hydrogen atom from their phenolic hydroxyl group to the radicals which were generated during the first step of degradation and reacted promptly with oxygen, creating hydroperoxides this way [3,4]. Sulphur or Phosphorus containing secondary stabilizers are applied as hydroperoxide decomposers, as these substances are able to reduce hydroperoxides to stable alcohols and water. Brocca [5] raised the attention to the possibility of diffusion of small molecular weight stabilizers from the polymer matrix; however, the effects of reaction products of these substances on human health are still unknown.
By the production of phenolic antioxidants, plants are efficiently protecting themselves against photooxidation initiated by ultraviolet radiation, which has similar aspects as thermooxidation. Curcumin or the members of flavonoids, for example quercetin or rutin proved their beneficial effects on human health and are applied by medicine for a long while.
The research goals, open questions
The aim of our work is to replace the above mentioned synthetic, phenolic stabilizers with natural antioxidants during the processing of polyolefins. Stabilizing efficiency of these antioxidants were compared to the frequently applied synthetic stabilizer Irganox 1010 [L5], but it turned out soon, that the examined antioxidants outperformed the reference stabilizer [6,B1]. Based on these early results, the direction of the work shifted toward the investigation of relationship between the molecular structure, the applied concentration and the efficiency of these natural substances. Changes in the properties of the polymer during its repeated processing were analyzed according to the industrial practice, and interactions between the applied primary and secondary stabilizers were characterized too.
Methods
Sample preparation:
We applied the Tipelin FS-471-02 grade HDPE powder [L6], obtained from TVK. The polymer was polymerized by Philips type catalyst [L7]. Different natural antioxidants (quercetin [6], dihydromyricetin [B3,B4], silymarin [B5] or rutin [B7]) were applied as primary stabilizers in 0-500 ppm concentration range, while we added 1000 ppm PEPQ [L8] as secondary stabilizer to the polymer. Powder mixtures were homogenized by a Henschel FM/A10 powder mixer at 500 rpm for 10 minutes. The mixtures were processed by six consecutive extrusion steps in a Rheomex S ¾” type single screw extruder connected to a Haake Rheocord EU 10V driving unit, with the following parameters: diameter: 4 mm; velocity: 50 rpm, zone temperatures: 180-220-260-260 °C. Products were cooled down by water bath, and then they were granulated. Samples were taken from the granulates after every extrusion steps. ~100 µm thin films were created for the spectroscopy measurements by a Fontijne SRA 100 laboratory press at 190 °C with 3 minutes preheating and 2 minutes of pressing times. Rheology, thermoanalytical and color measurements were carried out directly on the granulates.
Measurements:
Changes in the chemical structure of polyethylene (the concentration of unsaturated groups) and depletion of the secondary stabilizer during the extrusion steps were characterized by Fourier-transform infrared spectroscopy (FTIR) in transmission mode [L9]. The measurements were carried out on a Bruker Tensor 27 device applying 4000-400 cm-1 wavenumber range, 2 cm-1 resolution and 16 repeating time.
Changes of the viscosity of the polymer were characterized by the measurement of the melt flow rate (MFR) [L10] by a Göttfert MPS-D device. A metal piston with a constant 2.16 kg load is pulled through a preheated (190 °C) capillary filled with the polymer granulates during the measurement. The weight of the outpressed, melted material had been measured over a specified time, which is largely influenced by any changes of the average molecular weight of the polymer.
Residual thermooxidative stability of the samples were measured by the determination of their oxidation induction times (OIT) [L11] by a Perkin Elmer DSC 7 device. Samples were heated up to 190 °C and were placed in a continuous oxygen flow during the measurement. OIT is the time required for the appearance of the exothermic peak, which indicates the beginning of the oxidation of the sample.
Color of the samples was characterized by a Hunterlab Colorquest 45/0 device. Yellowness and average brightness of the samples were measured after each extrusion steps.
Results
Polyethylene chains polymerized by Philips type catalyst are ending always with a methyl and an unsaturated (vinyl) group; however, failures during the polymerization process lead to the formation of additional unsaturation in the molecule. Radicals generated randomly during the processing of PE react with these unsaturated groups the most easily, which phenomenon leads to the decrease of the concentration of these groups. Concentration of the unreacted secondary stabilizer is decreasing too, as this additive constantly reacts with the forming hydroperoxides [8]. Flow rate of the polymer samples is plotted against the number of extrusion steps in Figure 1, which shows a continuous decrease by increased number of extrusions, which means that the dominant degradation process is the formation of long chain branches. Based on the results we can conclude that dihydromyricetin efficiently hindered this process even at 25 ppm concentration until the fifth extrusion step.
Melt flow rate of the polymer samples is plotted against their residual secondary stabilizer concentration in Figure 2, which shows that the concentration of PEPQ decreases prompt by the radical chain reaction (indicated by the upper-right part of the graph), but this process leads to the formation of long chain branches and so to the increase of viscosity only after the depletion of the secondary stabilizer from the polymer.

Figure 1.: Melt flow rate of the polymer plotted against the number of extrusion steps

Figure 2.: Melt flow rate of the polymer plotted against the residual PEPQ concentration in the samples
Flavonoid type antioxidants react with free radicals similarly to the synthetic phenolic stabilizers: they donate a hydrogen atom from their phenolic hydroxyl group to the reactive radical and so stabilize it. The flavonoid backbone structure is represented in Figure 3. It is visible, that different flavonoids can be separated by the amount and location of their phenolic hydroxyl groups. We aimed to explore the connection between these parameters and the stabilizing efficiency of flavonoid substances.
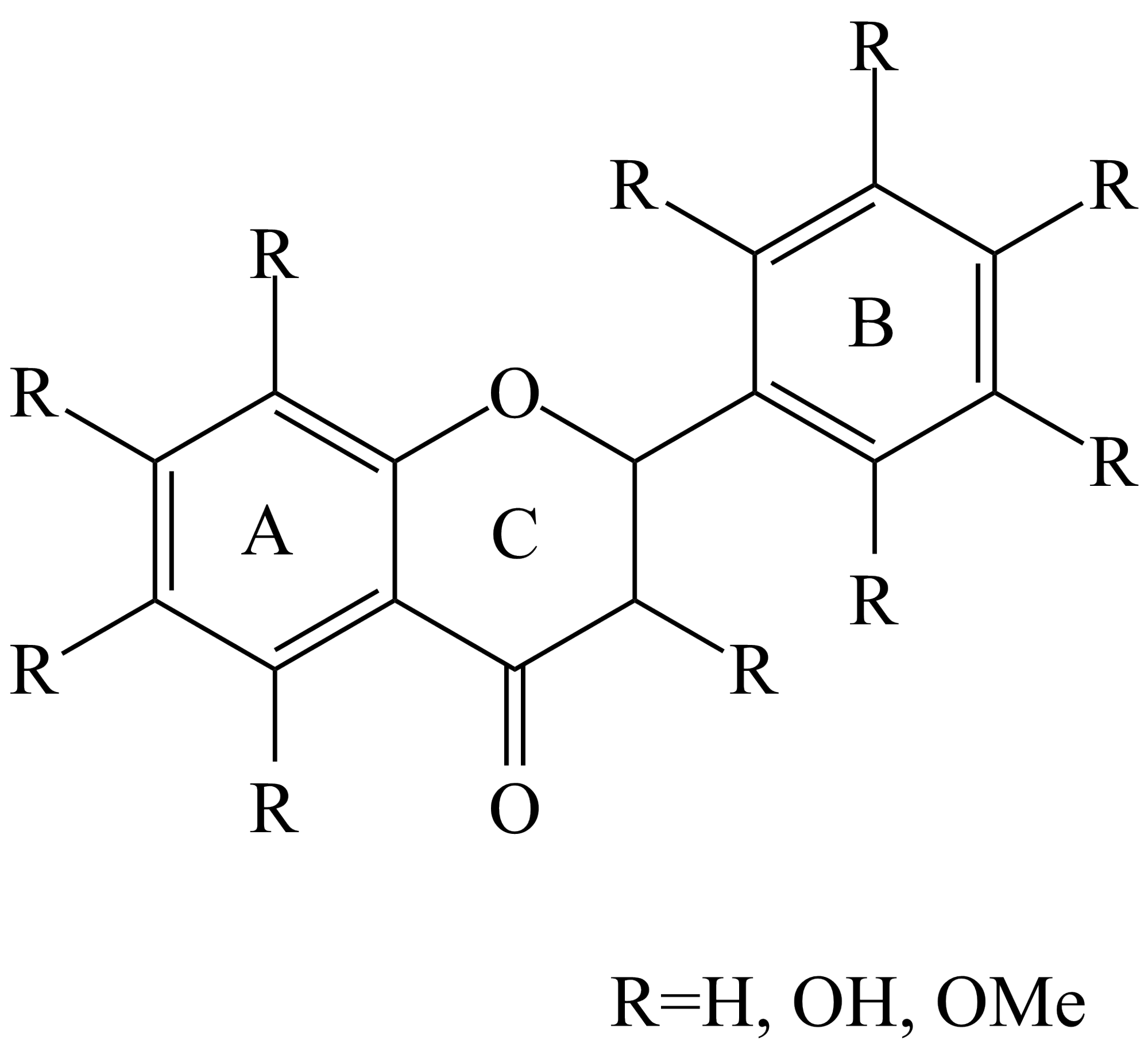
Figure 3.: Base molecular structure of flavonoids
OIT values of samples containing different types of flavonoid antioxidants were plotted against the applied concentration of the stabilizers on Figure 4 after the first extrusion step. It can be seen that the amount of phenolic hydroxyl groups of the different types of flavonoids is the determining factor of stabilizing efficiency, at least in the case of complete oxidation of the sample. Residual concentration of PEPQ is represented in Figure 5 in the case of samples containing the same amounts of natural antioxidants after the first extrusion step. The values are plotted against the lowest O-H bond dissociation energy (BDE) [L12] of the different flavonoids. The value of this property depends mostly on the chemical environment of the hydroxyl group. The lower the BDE values is, the more efficiently hinders the stabilizer the decomposition of PEPQ, which is highly important in order to maintain the mechanical properties of polyethylene.

Figure 4.: OIT values of samples containing different flavonoids, after the first extrusion step [B6]
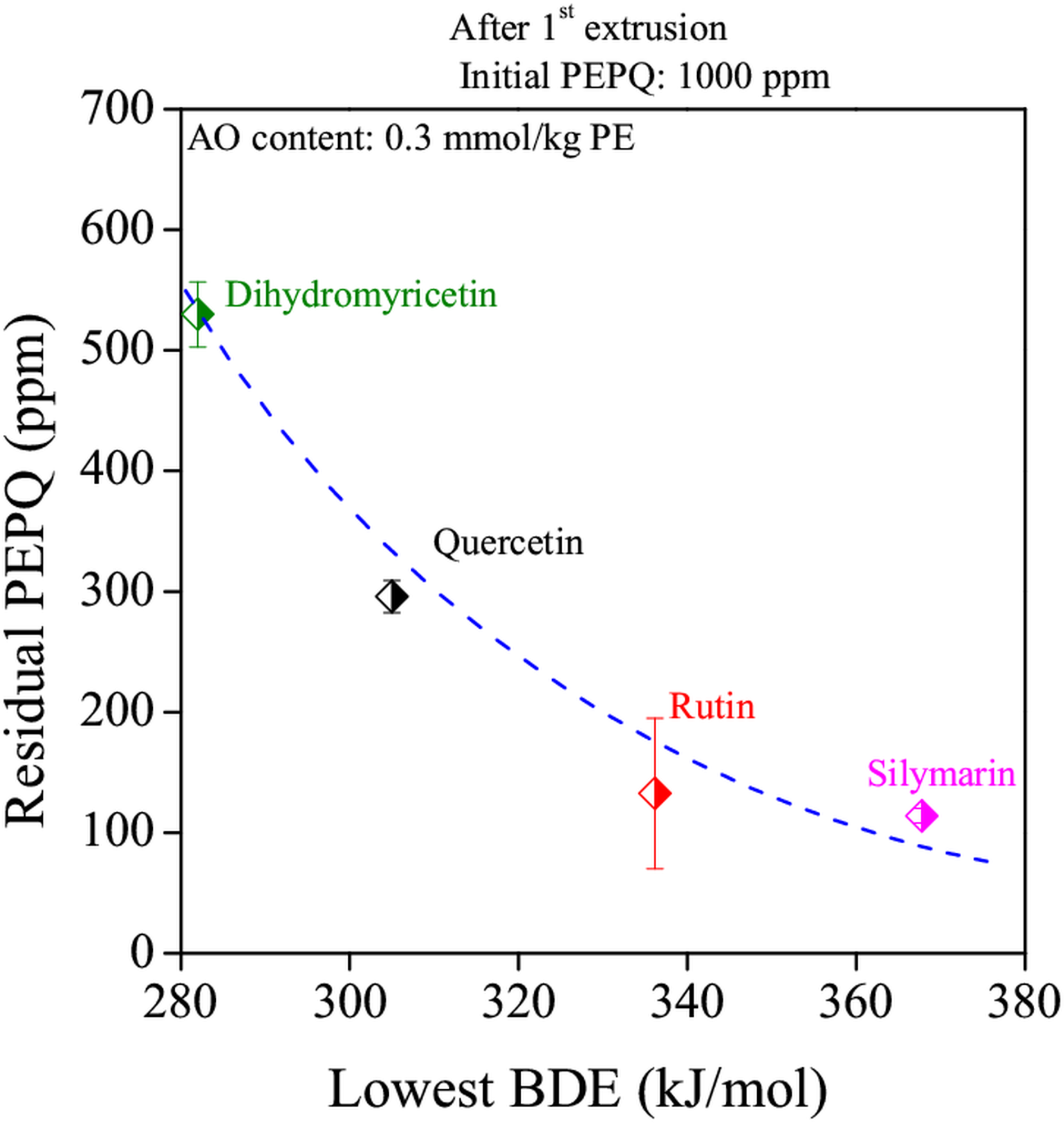
Figure 5.: Dependence of residual PEPQ concentration on BDE values of different flavonoids, after the first extrusion step [B6]
Expected impact and further research
Stabilizing efficiency of some flavonoids outperforms commonly applied synthetic, hindered phenolic stabilizers. Based on our results we can conclude that some of the connections between the efficiency and the molecular structure of flavonoids have been investigated. On the other hand, their severe coloring effect, varying purity and price emerge further obstacles before the industrial application of flavonoids. An additional direction of the research work is aiming at the development of secondary stabilizers from the nature, which could lead to a completely natural processing stabilization of polyolefins.
Publications, references, links
List of corresponding own publications:
B1. Tátraaljai, D., Kirschweng, B., Kovács, J., Földes, E., Pukánszky, B.: Processing Stabilization of PE with a Natural Antioxidant, Curcumin, European Polymer Journal, 1196-1203, 49, 2013, IF: 3.477
B2. Kirschweng, B., Tátraaljai, D., Földes, E., Pukánszky, B.: Efficiency of curcumin, a natural antioxidant, in the processing stabilization of PE: Concentration effects, Polymer Degradation and Stability, 17-23, 118, 2015, IF: 3.120
B3. Kirschweng, B., Bencze, K., Sárközi, M., Hégely, B., Samu, Gy., Hári, J., Tátraaljai, D., Földes, E., Kállay, M., Pukánszky, B.: Melt stabilization of polyethylene with dihydromyricetin, a natural antioxidant, Polymer Degradation and Stability, 192-200, 133, 2016, IF: 3.120
B4. Kirschweng, B., Bencze, K., Sárközi, M., Hári, J., Tátraaljai, D., Földes, E., Pukánszky, B.: Phillips típusú polietilén feldolgozási stabilizálása a dihidro-mirycetin természetes antioxidáns felhasználásával, Polimerek, 151-155, 3(5), 2017, no IF
B5. Kirschweng, B., Vörös, B., Tátraaljai, D., Földes, E., Pukánszky, B.: Natural antioxidants as melt stabilizers for PE: comparison of silymarin and quercetin, European Polymer Journal, 456-466, 90, 2017, IF: 3,477
B6. Kirschweng, B., Tátraaljai, D., Földes, E., Pukánszky B.: Natural antioxidants as stabilizers for polymers, Polymer Degradation and Stability, 25-40, 145, 2017, IF: 3.386
B7. Kirschweng, B., Tilinger, MD., Hégely, B., Samu, Gy., Tátraaljai, D., Földes, E., Pukánszky, B.: Melt stabilization of PE with natural antioxidants: Comparison of rutin and quercetin, European Polymer Journal, 228-237, 103, 2018, IF: 3.531
Summarized impact factor: 20.111
Table of links:
L1. https://en.wikipedia.org/wiki/Polyethylene
L2. https://en.wikipedia.org/wiki/Flavonoid
L3. http://www.mua.bme.hu/
L4. http://www.ttk.mta.hu/en/intezetek/anyag-es-kornyezetkemiai-intezet/polimerfizikai-osztaly/
L5. http://www.shanghaiguanan.com/pic/2014916113724268.pdf
L6. https://mol.hu/images/pdf/Vallalatiugyfeleknek/polimer_termekek/hdpe-kozepes-esnagysurusegu_polietilenek/fs-471-02_eng.pdf
L7. https://en.wikipedia.org/wiki/Phillips_catalyst
L8.https://www.palmerholland.com/Assets/User/Documents/Product/42144/320/MITM02915.PDF
L9 https://en.wikipedia.org/wiki/Fourier-transform_infrared_spectroscopy
L10 https://en.wikipedia.org/wiki/Melt_flow_index
L11 https://en.wikipedia.org/wiki/Oxidative-induction_time
L12 https://en.wikipedia.org/wiki/Bond-dissociation_energy
List of references:
1. Gol'dberg, V.M., Zaikov G.E.: Kinetics of mechanical degradation in melts under model conditions and during processing of polymers—A review, Polymer Degradation and Stability, 221-250, 19(3), 1987
2. Moss, S., Zweifel, H.: Degradation and stabilization of high density polyethylene during multiple extrusions, Polymer Degradation and Stability, 217-245, 25(2–4), 1989
3. Pospíšil, J.: Chemical and photochemical behaviour of phenolic antioxidants in polymer stabilization: A state of the art report, part II, Polymer Degradation and Stability, 103-115, 39(1), 1993
4. Pospíšil, J.: Chemical and photochemical behaviour of phenolic antioxidants in polymer stabilization—a state of the art report, Part I, Polymer Degradation and Stability, 217-232, 40(2), 1993
5. Brocca, D., Arvin, E., Mosbæk, H.: Identification of organic compounds migrating from polyethylene pipelines into drinking water, Water Research, 3675-3680, 36(15), 2002
6. Tatraaljai, D., Foldes, E., Pukanszky, B.: Efficient melt stabilization of polyethylene with quercetin, a flavonoid type natural antioxidant, Polymer Degradation and Stability, 41-48, 102(4), 2014
