|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Dr. Hazai László
Synthesis of Vinca alkaloid derivatives with anticancer activity
Introducing the research area
Our research deals with the famous Vinca alkaloid family [1,2]. These natural compounds can be isolated from the leaves of Catharanthus roseus.

Figure 1. The source of Vinca alkaloids: the periwinkle plant Catharanthus roseus
Some of these active substances along with their semisynthetic analogs are still being used as chemotherapeutic agents in anticancer therapy (especially in case of lymphomas and leukemia). Cancer cells are derived from healthy cells. Due to a carcinogen impact (chemical substances, radiation, virus or sunlight), the DNA of healthy cells could be altered, leading to cellular degeneration through uncontrolled signaling processes (unrestricted reproduction). The basic aim of our research project is to synthesize new Vinca alkaloid derivatives by connecting them with various pharmacophore units in order to increase their effectiveness and/or reduce their serious toxicity.
Brief introduction of the research place
The place of our research is the Alkaloid Chemistry Group at the Department of Organic Chemistry and Technology. Our mission is to synthesize new derivatives with anticancer activity and to evaluate their cytotoxicity. This project unites preparative organic chemistry and medicinal chemistry through the assessment of structure-activity relationships. This complex workflow brings the beauty of our work: we are developing not only drug substances via organic synthesis but also investigate their biological activity. Prof. Dr. Csaba Szántay (1928-2016) - past honorary member of the Hungarian Academy of Sciences and also a renowned professor dealing with Vinca alkaloids - was a chemist and former supervisor of our research group. He made the breakthrough on developing vinpocetine, the active substance of Cavinton®. It is still used for enhancing memory and preventing Alzheimer’s disease since it could improve blood flow to the brain.

Figure 2. The former leader and excellent researcher of Alkaloid Chemistry Group at BUTE, Dr. Csaba Szántay (1928–2016), who synthesized vinpocetine first. It is still on the market under the brand name Cavinton®
History and context of the research
Alkaloids are a class of naturally occurring organic compounds that mostly contain basic nitrogen atoms. They have no unified structure, the family includes morphine, nicotine, atropine, quinine, papaverine, and cocaine, among others. Thus these natural compounds have a wide range of pharmacological activities. Vinca alkaloids were discovered in the 1950s [3]. The dimer alkaloids vinblastine (1) and vincristine (2), isolated from the Madagascar periwinkle Catharanthus roseus are remarkable representatives of the bisindole alkaloid family. Their complex structure consists of two subunits with an indole skeleton: catharanthine (3) and vindoline (4).
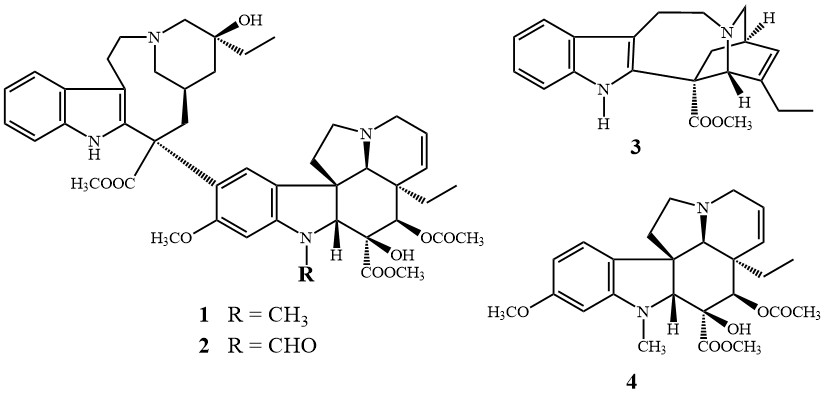
Figure 3. The alkaloids vinblastine (1), vincristine (2), catharanthine (3) and vindoline (4)
The latter are present in the plant in much larger quantities than dimers (0.005%), but do not have anti-cancer effects on their own. Therefore, the growing interest and demand for Vinca alkaloids in the pharmaceutical industry have been met not only by isolation from the plant but also by the synthetic linking of monomers and even by total synthesis. Interestingly, vincristine (2) was examined first thinking it could have an antidiabetic effect and while the alkaloid was being analyzed in animals it turned out that it caused granulocytopenia (reduction in the number of white blood cells) and the repression of the marrow. It was also discovered that the hypoglycemic activity had little importance compared to its cytotoxic effect.

Figure 4. Pharmaceutical products containing vinblastine (1) and vincristine (2)
The anticancer activity of Vinca alkaloids stems from their prevention of microtubule polymerization [4]. Microtubules are components of the cytoskeleton and are made up of two monomer proteins called α- and β-tubulin. They are not only involved in maintaining the structure of the cellular division but also contribute to the construction of mitotic spindles that control and facilitate the detachment of chromosomes through mitosis and meiosis. These drugs can inhibit the differentiation of cancer cells, which leads to their death. It is not a coincidence that they are referred to as anti-mitotic alkaloids as well. Vinca alkaloids are usually used in the form of sulfates salts and are administered via intravenous injection in clinical therapy since their absorption is poor from the gastrointestinal tract. Besides their significant side effects (neurotoxicity, myelosuppression), multidrug-resistance (MDR) there is another problem that restricts the applicability of these pharmaceutical molecules in clinical therapy. The key protein involved in MDR is P-gp (permeability glycoprotein), which has many endogen and exogen substrates including Vinca alkaloids. P-gp is an ATP-dependent efflux pump and a remarkable member of the ATP-binding cassette family (ABC transporter) possessing broad substrate specificity. This protein is responsible for pumping several drugs out of the cell, e.g. Vinca alkaloids among others before they can exert their influence inside.
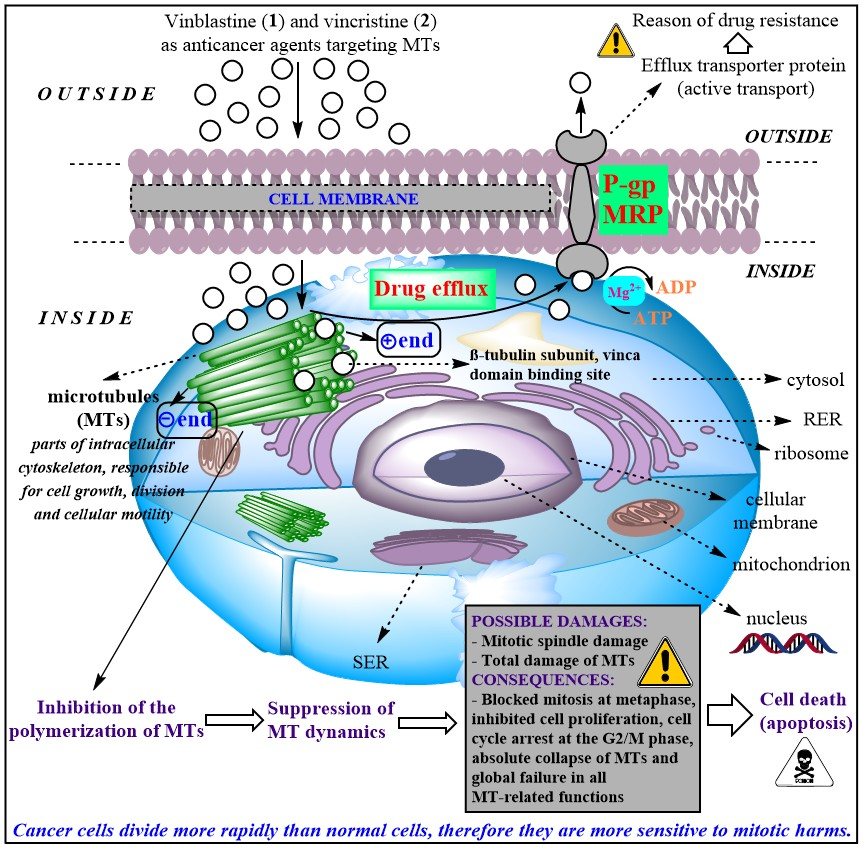
Figure 5. Vinblastine (1) and vincristine (2) as they disrupt the normal function of microtubules that subsequently lead to the death of (primarily tumor) cells. P-gp: P-glycoprotein. MRP: multidrug resistance-related protein. RER: rough endoplasmic reticulum. SER: smooth endoplasmic reticulum
The research goals, open questions
An important approach in the development of selective anticancer drugs is the synthesis of special complex molecules, called “hybrids” [5,6]. A hybrid drug comprises the incorporation of two drug pharmacophores in one single molecule which is designed to interact with multiple targets or to amplify its effect through action on another bio target as one single molecule or to counterbalance the known side effects associated with the different parts. A pharmacophore is a separable, biologically active structural unit (with a defined configuration) within a molecule that can be recognized by one or more receptors. In hybrid structures, the subunits are always covalently bound. The term “hybrid” refers to natural or originally natural compounds conjugated with (semi)synthetic compounds possessing at least two pharmacophores in one molecule. Building hybrid molecules is a well-known and widespread method in modern drug investigation, especially in the field of oncology. Our group has previously produced some Vinca derivatives linked to amino acids [7] and steroids. The great advantage of linking with amino acids is that the given products bound to carrier peptides (e.g. octaarginine) are able to directly enter the cell, thereby enabling more targeted therapy and thus reducing the serious side effects. On the other hand, the steroid vector may facilitate the internalization of the drug into the cell. The obtained vindoline-steroid hybrid products had promising biological activities.
Methods
After the above mentioned encouraging experiments, attempts have been made to obtain Vinca alkaloid conjugates coupled with synthetic, generally known pharmacophores, such as morpholine, piperazine, N-methylpiperazine and triphenylphosphine [8,9]. The experiments were performed starting from vindoline (4). This monomer does not show anticancer activity in itself, however, it was supposed, that its efficacy could be promoted by linking it to synthetic pharmacophore units.

Figure 6. The structure of the synthetic pharmacophore units to be attached to Vinca alkaloids
The methods used in practice include the known techniques applied in the process of organic chemical preparatory work, in compliance with the appropriate safety considerations. Preparative thin-layer chromatography is generally used to purify our compounds. The structure identification is performed by nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). Recording and evaluation of NMR and MS spectra are carried out by Gedeon Richter Plc. (Structural Research Department).
Results
In the first step in the synthesis of Vinca alkaloid hybrids, 17-desacetylindoline had to be produced from vindoline (4) by ester hydrolysis, after which the compound could be linked to different bromocarboxylic acids. It was assumed that the chain length of the linkers could also affect the antitumor effect of the whole molecule. After the linker containing 17-desacetylvindoline derivatives (5,6) were synthesized, the next step was to couple them with the synthetic pharmacophores. First, the coupling reaction with morpholine was tested. The synthetic work was successful, but biological studies revealed that this derivative had no significant antiproliferative effect. Couplings with piperazine were then studied. In this case, a dimer product was obtained, namely a vindoline unit with 1-1 linkers attached to both sides (N atoms) of piperazine. The preparation of a functionalized derivative only on one side of piperazine was accomplished using N-methylpiperazine. Biological evaluation of the latter two derivatives is still ongoing. Finally, we have prepared vindoline derivatives coupled with triphenylphosphine. We wanted to test phosphorus-containing structural units as possible coupling components, and we hoped that triphenylphosphine could improve cell membrane permeability and promote the accumulation of the drug within the cell. In addition, it has been found in the literature [9] that triphenylphosphine itself has cytotoxic activity (cytotoxic = causing cell death; cytostatic = causing the inhibition of cell growth and multiplication). Coupling reactions with triphenylphosphine resulted in the expected phosphonium-salts (7,8). In our related experiments, vindoline derivatives 5 and 6 were dissolved in absolute (anhydrous) acetonitrile under argon, and the reaction mixtures were heated (refluxed) to give the two phosphonium salts (7,8).

Figure 7. Synthesis of the Vinca alkaloid - triphenylphosphine hybrid molecules (7,8)
The expected conjugated derivatives were successfully synthesized. Furthermore, some promising results were already observed by the National Institutes of Health (NIH), US, where the anticancer activities were tested on 60 different cell lines in vitro. The results of the first studies showed that vindoline coupled with only a linker (4-bromobutyric acid) was ineffective, and the morpholine-linked derivative was not significantly effective either. However, derivatives coupled with triphenylphosphine (7,8) showed such good results that, following one-dose screen, NIH subjected them to further testing against the 60 cell panel at five concentration levels (five-dose screen). The assessment resulted in GI50 values. The NCI (National Cancer Institute) renamed the IC50 value as the concentration that causes 50% growth inhibition (GI50). The GI50 measures the growth inhibitory power of the test agent and the values emphasize the correction for the cell count at time zero [10]. Based on the given GI50 values, triphenylphosphine-containing hybrid molecules (7,8) proved to be much more effective than vindoline (4), but did not achieve the anti-cancer activity of vinblastine (1). These results suggest that Vinca alkaloid monomers may become potential anticancer agents in a hybrid form.
Expected impact and further research
In our current project, we studied various pharmacophore hybridization experiments and successfully produced vindoline derivatives linked to morpholine, piperazine, N-methylpiperazine, and triphenylphosphine. A demonstrable antitumor effect was presumed by connecting a Vinca alkaloid monomer to synthetic pharmacophore units. The biological activities of some of the new compounds were tested at the NIH, US. Of these, triphenylphosphine derivatives (7,8) had significant antiproliferative effects with outstanding GI50 values in several tumor cell lines that confirmed the expected positive role of the triphenylphosphine unit. In the future, we still plan to produce Vinca alkaloid hybrids with new pharmacophores. In addition, we would like to strengthen our collaboration with the research groups of Dr. Ferenc Hudecz (ELTE) and Dr. Attila Hunyadi (SZTE) in order to extend the biological studies.
List of publications.
1) Natural compounds containing a condensed cyclopropane ring. Natural and synthetic aspects. Current Organic Chemistry, 2014, 18, 2037–2042. IF: 2.193
https://doi.org/10.2174/1385272819666140721190257
Authors: Keglevich, P.; Keglevich, A.; Hazai, L.; Kalaus, G.; and Szántay, C. . .
2) Anomalous Products in the Halogenation Reactions of Vinca Alkaloids. Current Organic Chemistry, 2016, 20, 2639–2646. IF: 2.193
https://doi.org/10.2174/1385272820666160617080202
Authors: Keglevich, A.; Hegedűs, L.; Péter, L.; Gyenese, J.; Szántay, C.Jr.; Dubrovay, Z.; Dékány, M.; Szigetvári, Á.; Martins ,A.; Molnár, J.; Hunyadi, A.; Keglevich, P. and Hazai, L.
3) The effect of conjugation on antitumor activity of vindoline derivatives with octaarginine, a cell‐penetrating peptide. Journal of Peptide Science, 2018, 24, 3118. IF: 1.969
https://doi.org/10.1002/psc.3118
Authors: Bánóczi, Z.; Keglevich, A.; Szabó,I.; Ranđelović, I.; Hegedüs, Z.; Regenbach, F.L.; Keglevich, P.; Lengyel, Z.; Gorka-Kereskényi, Á.; Dubrovay, Z.; Háda, V.; Szigetvári, Á.; Szántay, C.Jr.; Hazai, L.; Tóvári, J. and Hudecz, F.
4) Attempted Synthesis of Vinca Alkaloids Condensed With Three-Membered Rings. Molecules, 2018, 23, 2574. IF: 3.098
https://doi.org/10.3390/molecules23102574
Authors: Keglevich, A.; Mayer, S.; Pápai, R.; Szigetvári, Á.; Sánta, Z; Dékány, M.; Szántay C.Jr.; Keglevich, P. and Hazai, L.
5) A mainly NMR-based structure elucidation of a surprising vindoline trimer with the aid of non-uniform sampled 1H-13C HSQC and HMBC spectra. Structural Chemistry, 2019, 30, 795–804. IF: 2.019
https://doi.org/10.1007/s11224-018-1267-1
Authors: Szigetvári, Á.; Keglevich, A.; Keglevich, P.; Dékány, M.; Hazai, L. and Szántay, C.Jr.
6) Synthesis of Vinca alkaloid – triphenylphosphine derivatives having potential antitumor effect. Phosphorus, Sulfur, and Silicon and the Related Elements, 2019, 194, 606-609. IF: 0.674
https://doi.org/10.1080/10426507.2018.1550780
Authors: Keglevich, A.; Szigetvári, Á.; Dékány, M.; Szántay, C.Jr.; Keglevich, P. and Hazai, L.
Further, already accepted publications.
7) Synthesis and in vitro Antitumor Effect of New Vindoline Derivatives Coupled with Triphenylphosphine. Current Organic Chemistry, 2019, 23, 1-7. IF: 2.193
Authors: Keglevich, A.; Szigetvári, Á.; Dékány, M.; Szántay, C.Jr.; Keglevich, P. and Hazai, L.
8) Synthesis and in vitro Antitumor Effect of New Vindoline-Steroid Hybrids. Current Organic Chemistry, 2019, 23, 1–9. IF: 2.193
Authors: Keglevich, A.; Zsiros, V.; Keglevich, P.; Szigetvári, Á.; Dékány, M.; Szántay, C.Jr.; Mernyák, E.; Wölfling, J. and Hazai, L.
Additional, already accepted, short communications.
9) Vinca alkaloidok és rákkutatás (informative article competition, third award, 2019). Élet és Tudomány. IF: -
Authors: Keglevich, A.
Table of links.
structure-activity relationships
P-gp (permeability glycoprotein)
nuclear magnetic resonance (NMR) spectroscopy
References.
[1] Arora, R.; Malhotra, P.; Mathur, Ajay K.; Mathur, Archna; Govil, C.M.; Ahuja, P.S. Anticancer Alkaloids of Catharanthus roseus: Transition from Traditional to Modern Medicine. Herbal Medicine: A Cancer Chemopreventive and Therapeutic Perspective, 2009, 21, 292–310.
[2] Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284.
[3] Moudi, M.; Go, R.; Yong Seok Yien, C.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013; 4, 1231–1235.
[4] Ngan, V.K.; Bellman, K.; Hill, B.T.; Wilson, L.; Jordan, M.A. Mechanism of mitotic block and inhibition of cell proliferation by the semisynthetic Vinca alkaloids vinorelbine and its newer derivative vinflunine. Mol. Pharmacol. 2001, 60, 225–32.
[5] Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487.
[6] Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem., 2016, 124, 500–536.
[7] Keglevich, P.; Hazai, L.; Gorka-Kereskényi, Á.; Péter, L.; Gyenese, J.; Lengyel, Zs.; Kalaus, Gy.; Dubrovay, Zs.; Dékány, M.; Orbán, E.; Szabó, I.; Bánóczi, Z.; Szántay, Cs. Jr.; Szántay, Cs. Synthesis and in vitro Antitumor Effect of New Vindoline Derivatives Coupled with Amino Acid Esters. Heterocycles 2013, 87, 2299–2317.
[8] Al-Ghorbani, M.; Bushra, Begum A.; Zabiulla, S.; Mamatha, S.V.; Ara Khanum, S. Piperazine and morpholine: Synthetic preview and pharmaceutical applications. J. Chem. Pharm. Res., 2015, 7, 281–301.
[9] Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong H.H.; Mironov, V.F. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. J. Nat. Prod. 2017, 80, 2232–2239.
[10] National Institutes of Health, National Cancer Institute, Division of Cancer Treatment & Diagnosis, Developmental Therapeutics Program, 2019.
https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm
https://dtp.cancer.gov/databases_tools/docs/compare/compare_methodology.htm
