|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Applied Biotechnology and Food Science
Supervisor: Dr. Vértessy G. Beáta
Quantitation of DNA-building Nucleobases to set up a Model of Antibiotic Resistance Development
Introducing the research area
DNA replication accuracy has a great importance in maintaining the life of a cell. The four nucleobases (guanine: dGTP, cytosine: dCTP, thymine: dTTP, adenine: dATP, collectively dNTPs) are maintained in fine-tuned balance and quantity within the cell. This precise balance contributes to accurate DNA replication, while an off-balance stimulates the production of DNA errors (mutations)1-3. Therefore, a change in the levels of nucleobases contributes greatly to the development of antibiotic resistance. In my research, I study the quantities of nucleobases in a bacterial environment to explore the mechanisms of antibiotic resistance formation, thereby allowing the development of better antibiotics.
Brief introduction of the research place
I work at the BUTE VBK Department of Applied Biotechnology and Food Science in the ‘Genome metabolism and DNA repair’ research group led by Prof. Dr. Vértessy G. Beáta. The main profile of the group is studying DNA errors and enzymes involved in the reparation of these errors, including the study of antibiotic resistance development as well.
History and context of the research
Many bacterial infections have become curable after the discovery of antibiotics. However, over more than half a century of excessive and sometimes unnecessary use of antibiotics have made the treatment of these infections a challenge, because initially effective antibiotics are gradually becoming ineffective due to the emerging antibiotic resistance. There is no difference in the case of Mycobacterium tuberculosis: the long treatment duration and the excessive use of antibiotics have led to the development of multiresistant strains4, carrying genes resistant to more than one drugs. Moreover, there is a threat from those strains, which are also resistant against the newly developed antibiotics (extremely resistant strains). Since the members of the Mycobacterium genus develop antibiotic resistance genes exclusively by series of point mutations5, therefore, it can be an excellent model organism to study the development of antibiotic resistance, as opposed to those organisms which can get the resistance gene from their environment or from other bacteria. In my research I study the intracellular emergence of antibiotic resistance in Mycobacterium organism. My aim is to set up a model that can predict the development of antibiotic resistance, even for the medicines under development, thus allowing designing better medications.
The research goals, open questions
Since the bacteria carrying resistance genes are developing these genes under the pressure of antibiotics in a selective stress environment, I aim to study the relevant stress factors in the life cycle of Mycobacterium tuberculosis that may contribute to the increased mutation ability (Figure 1). This bacterium spreads in aerosol droplets in the air and also keeps its virulence for a long time in dry circumstances. Thus the environmental stress factors for the Mycobacterium tuberculosis are the UV radiation and starvation. When it infects a host, it can survive despite the harsh conditions, and it is able to reproduce itself even if it was taken up by immune cells. Therefore, the bacterium in a host is affected by the following stress factors: dormancy, alkylation, reactive oxygen species, and nitrogen radicals. If the host is treated by antibiotics, the first-line and second-line antibiotics also become stress factors for the bacterium, against which it has to struggle for survival. I systematically study how these identified stress circumstances having a mutation effect on the bacterium, enhance the development of resistance.
Antibiotics in general inhibit a vital process in the functioning of bacterial cells, so the resistance emerges as a change to the process coding gene. The alteration of genes is highly affected by the available building blocks during DNA replication; the shift in the quantity of nucleobases largely contributes to the mutation events6, thus my aim is to measure these nucleobase quantities separately for each mentioned stress effects (Figure 1). By studying each stress individually, those tendencies are becoming apparent that push the bacterium to adapt to the antibiotic environment with a resistance gene. I also determine the mutation pattern of each stress factors by identifying mutations in the genome (Figure 1), which is performed by the state-of-the-art, new generation full genome sequencing. I study the types of mutations corresponding to the shift in the nucleobase quantities for each stress effect. Therefore, my aim is to provide data for a model capable of predicting the probability and expected duration of the emergence of drug resistance for an antibiotic under development based on its mechanism.
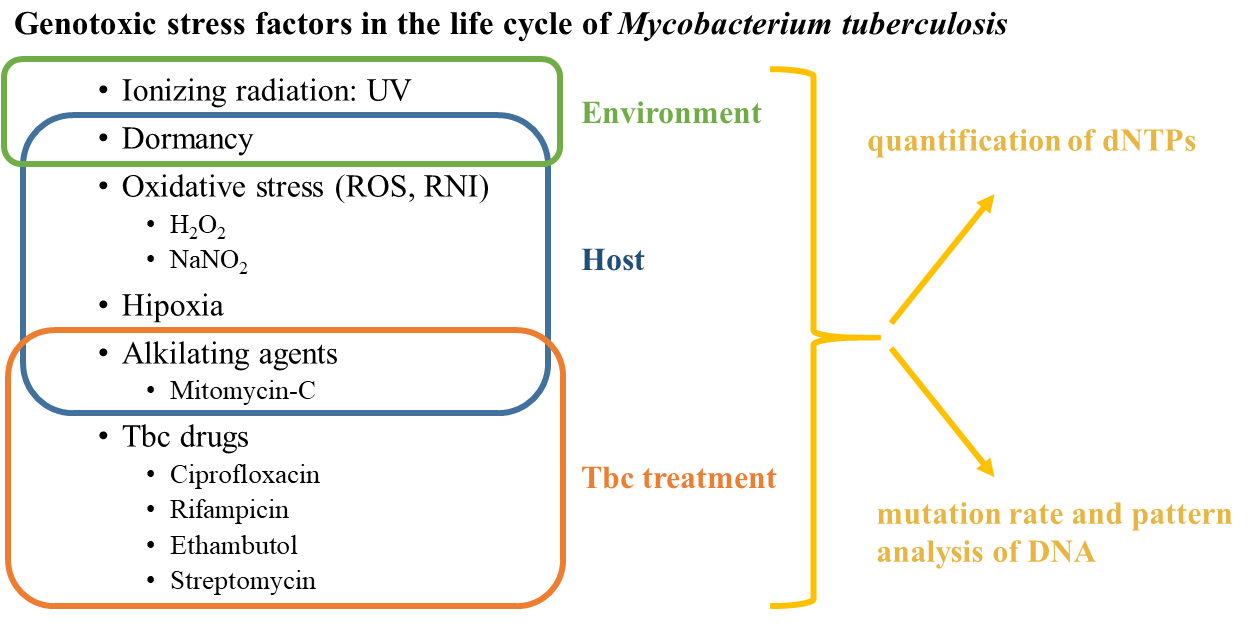
Figure 1: Important stress factors can contribute to antibiotic resistance genes development in Mycobacterium tuberculosis, therefore I aimed to investigate these factors and their effect.
Methods
Extraction of Mycobacterial nucleobases
The extraction of cellular small molecules (like nucleobases) is performed by methanolic extraction7: the Mycobacterium culture grown in broth is collected by centrifugation, then 1*109 cells are suspended in 500 μl 60% methanol. The small target molecules are dissolved in methanol, while the residual non-dissolving parts (cellular debris, denatured proteins, and DNA) are removed by another centrifugation step. The so-prepared pure methanol extract is vacuum-dried and dissolved in water.
Quantitation of DNA-building nucleobases
For the quantitative measurement of nucleobases in our laboratory we used a method described in the literature8, which is based on the measurement of fluorescent signal generated by the incorporation of nucleobases during DNA synthesis (Figure 2). This method provides an opportunity to measure the quantity of the four nucleobases individually. The measurement uses a single stranded DNA, which is a template for the newly synthesizing strand of the DNA double helix. In Figure 2, the method is demonstrated via the measurement of thymine (dTTP). A perfectly matching complementary primer DNA is hybridizing to the end of the template DNA, the DNA synthesis starts at the end of the primer. The DNA synthesis is performed by the DNA polymerase enzyme, which has the same role in the mitosis of organisms: it completes a given DNA template with another, complementary DNA strand. The DNA-building nucleobases we seek to measure are required for this new DNA strand. In the example, to complement the DNA with a new strand, six thymines are needed and when they are all incorporated, then the Taq DNA polymerase enzyme reaches the other end of the template, where a fluorescently tagged probe hybridizes. The fluorescence signal on the probe is inhibited by quencher molecules, so it does not provide a signal. However, the DNA polymerase enzyme can cut the probe chemically, and the fluorescent signal (in this case FAM) become spatially separated from the quencher molecules (ZEN, IBFQ) and generates the signal. This signal will be proportional to the quantity of six thymines. By measuring a calibration set, the quantity of thymine in the samples can be determined. The original form of the method, which is based on endpoint fluorescence detection, was not applicable on biological samples, due to the sample matrix influence. Therefore, we developed this method further with my colleagues: using fluorescence data taken from the entire duration of the measurement, we measure the quantity of incorporated nucleobases by a kinetic analysisS1. I developed an analysis software to facilitate data evaluation with this improved method – this allowed much easier and faster, and more straightforward data analysis.
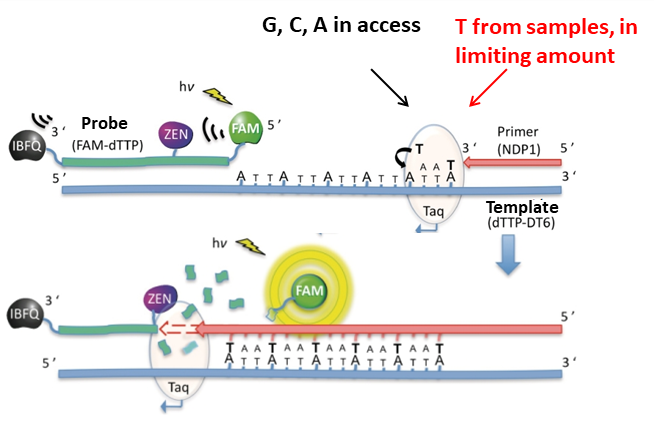
Figure 2: The method of quantifying DNA-building nucleobases via fluorescence signal. This figure illustrates the measurement of T base.
Mutation analysis of Mycobacterial genome
The whole genome sequencing of the Mycobacterium was performed by a company specialized in this field. However, the bioinformatic data analysis of the results was accomplished by me. By aligning the “reads” from the sequenced genome with the reference genome, several regions with a mismatching base could be identified: by collecting these variations, the mutation pattern could be determined.
Results
In my research, I concluded that stress treatments on the Mycobacterium induce changes in the quantity of DNA building nucleobases and the propensity of the bacterium to mutate. These variations differed by distinct treatments (Figure 3): while ciprofloxacin, rifampicin and streptomycin antibiotics increased the quantity of nucleobases and also changed their ratio, in the case of NaNO2 treatment these quantities decreased. However, in case of dormancy, the amounts of DNA-building nucleobases were not changing. Interestingly, UV irradiation resulted in an increase of one magnitude in the quantity of nucleobases, and the same applied to the oxidative stress represented by H2O2 treatment. On the other hand, the thymine (dTTP) synthesis inhibitor 5-fluorouracil (5-FU) selectively decreased the intracellular dTTP quantity, while the level of necessary cytosine precursor elevated. The quantity and quality of the stress-induced mutations were consistent with these results (Figure 4). In the case of 5-FU, the ‘C≡G → A=T’ the mutation frequency decreased, which is consistent with the increased cytosine level. The increase of the ‘T=A → G≡C’ mutation is considered to be the mutagenic effect of the ratio of a lower adenine level and a higher cytosine level. Compared to this, the ciprofloxacin, which inhibits DNA double helix winding and unwinding, cause significant increase in insertion and deletion mutations. In case of the streptomycin treatment, the mutation frequency did not increase, but on the contrary: less ‘C≡G → A=T’ mutation and less deletion happened compared to the non-treated cells. The H2O2 treatment caused an increase in the number of ‘C≡G → T=A’, ‘T=A → A=T’ and ‘C≡G → G≡C’ mutations, where the latest is obviously caused by the further oxidation of the oxidized guanine base9.
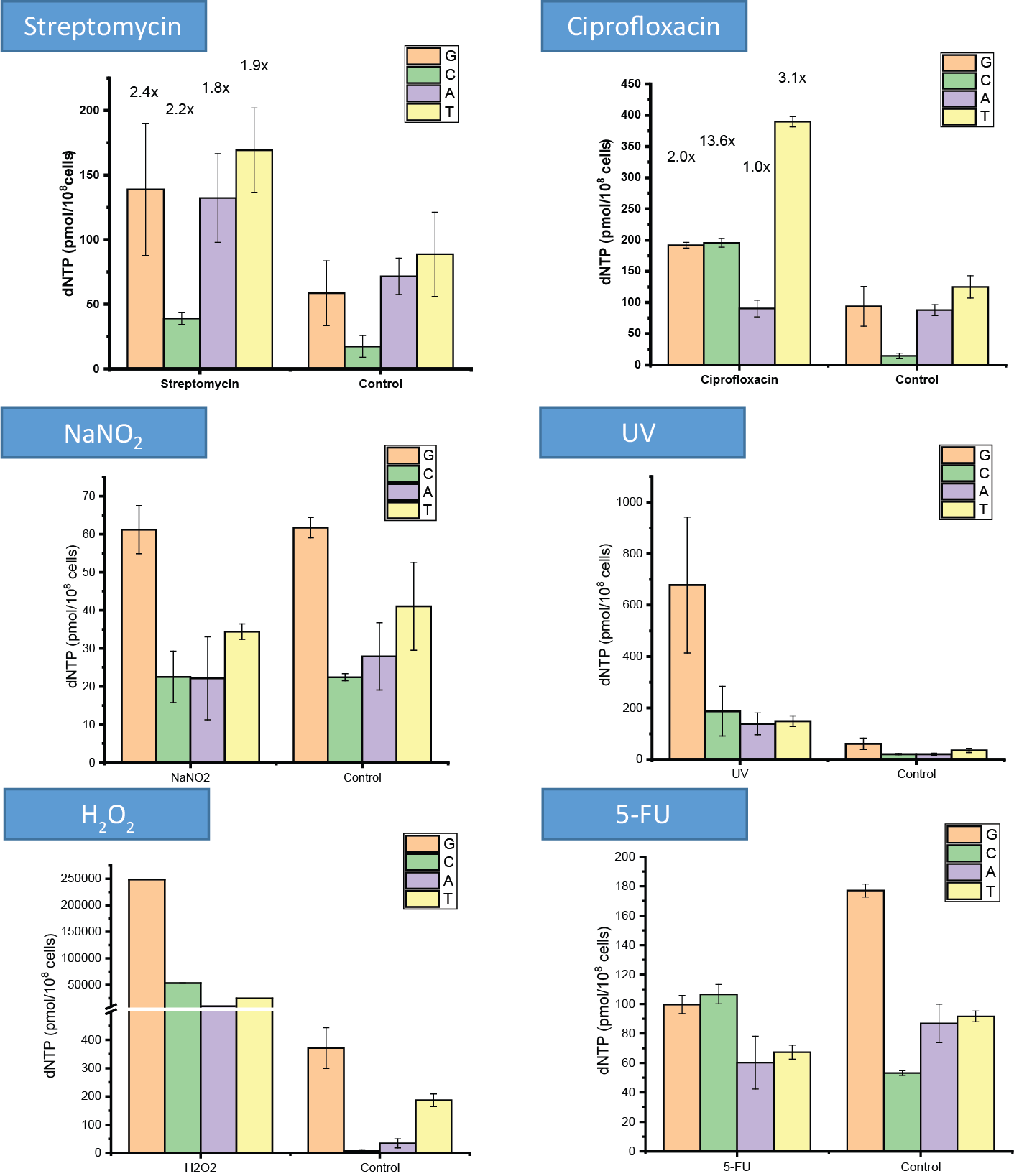
Figure 3: The quantity of DNA-building nucleobases under several different stress treatments
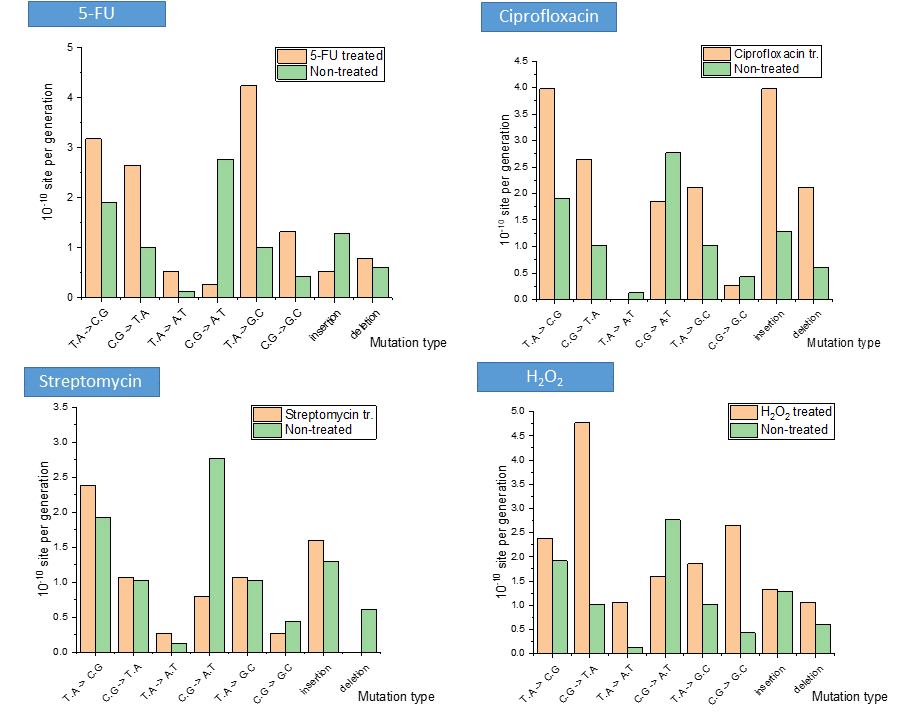
Figure 4: Changes in the basal mutation pattern upon several stress treatments
Expected impact and further research
As a part of my PhD research, 3 international refereed papers (S2-S5) and one paper in a Hungarian journal (S5) were published. Three further papers are under preparation and close to being submitted to journals. The results of my research on the antibiotic resistance of Mycobacteria can not only help the treatment of contagious Mycobacterium tuberculosis, but it can be useful in the treatment of many other bacterial infections. My colleagues include my results into a model, which incorporates the most important life processes of Mycobacterium tuberculosis bacterium, so it becomes possible to identify the weak points of the bacterium, like certain enzymes. Against the antibiotics targeted to these weak points, no antibiotic resistance development is expected to develop at all, or just very rarely, provided the role of the target is crucial in cell function.
Publications, references, links
List of corresponding own publications:
S1. J. E. Szabó*, É. V. Surányi*, B. Mébold, T. Trombitás, M. Cserepes, and J. Tóth, “A tool for the precise quantitation of deoxyribonucleoside triphosphates from biological samples”. Manuscript under submission (2019).
*First authors
S2. R. Hirmondó*, A. Lopata*, É. V. Surányi*, B. Vértessy G., and J. Tóth, “Differential control of dNTP biosynthesis and genome integrity maintenance by the dUTPase superfamily enzymes,” Sci. Rep.,7 (2017).
*First authors
S3. É. Surányi et al., “Exploiting a Phage-Bacterium Interaction System as a Molecular Switch to Decipher Macromolecular Interactions in the Living Cell,” Viruses, 10, 168, (2018).
S4. A. Benedek, F. Temesváry-Kis, T. Khatanbaatar, I. Leveles, É. V. Surányi, J. E. Szabó, L. Wunderlich, B. G. Vértessy, “The Role of a Key Amino Acid Position in Species-Specific Proteinaceous dUTPase Inhibition.” Biomolecules 9, 221 (2019).
S5. É. V. Surányi, R. Hirmondó, K. Nyíri, J. Tóth, and G. B. Vértessy, “Egy új molekuláris kapcsoló rendszer létrehozása élő sejten belüli makromolekuláris felismerés megfejtésére,” XV. PEME Konf. kiadvány, II, 130–135, (2017).
List of unrelated own publications:
S6. N. Z. Kiss, É. Böttger, L. Drahos, and G. Keglevich, “Microwave-Assisted Direct Esterification of Cyclic Phosphinic Acids,” Heteroat. Chem., 24, 283–288 (2013).
S7. N. Z. Kiss, Z. Mucsi, É. Böttger, L. Drahos, and G. Keglevich, “A Three-Step Conversion of Phenyl-1 H -phosphinic Acid to Dialkyl Phenylphosphonates Including Two Microwave-Assisted Direct Esterification Steps,” Curr. Org. Synth., 11, 767–772 (2014).
S8. N. Z. Kiss, Z. Mucsi, Z. Rádai, É. V. Böttger, and G. Keglevich, “The synthesis and potential use of cyclic phosphinic acid derivatives,” Phosphorus. Sulfur. Silicon Relat. Elem., 190, 668–671 (2015).
Table of links:
www.biostruct.org
http://www.biostruct.org/index.php/research/projects#dNTP
https://hu.wikipedia.org/wiki/Mycobacterium_tuberculosis
https://en.wikipedia.org/wiki/Reactive_oxygen_species
https://en.wikipedia.org/wiki/Reactive_nitrogen_species
https://en.wikipedia.org/wiki/Whole_genome_sequencing
http://nucleotidy.enzim.ttk.mta.hu/
List of references:
- D. Ahluwalia, R. J. Bienstock, and R. M. Schaaper, “Novel mutator mutants of E. coli nrdAB ribonucleotide reductase: insight into allosteric regulation and control of mutation rates,” DNA Repair (Amst)., 11,480–487 (2012).
- J. a Hollenbaugh et al., “dNTP pool modulation dynamics by SAMHD1 protein in monocyte-derived macrophages,” Retrovirology, 11, 63, (2014).
- P. Y. Ke, Y. Y. Kuo, C. M. Hu, and Z. F. Chang, “Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability,” Genes Dev., 19, 1920–1933 (2005).
- E. A. Kendall, A. S. Azman, F. G. Cobelens, and D. W. Dowdy, “MDR-TB treatment as prevention: The projected population-level impact of expanded treatment for multidrug-resistant tuberculosis,” PLoS One, 12, p. E0172748 (2017).
- M. Mcgrath, N. C. Gey van Pittius, P. D. van Helden, R. M. Warren, and D. F. Warner, “Mutation rate and the emergence of drug resistance in Mycobacterium tuberculosis,” J. Antimicrob. Chemother., 69, 292–302 (2014).
- S. Gon, R. Napolitano, W. Rocha, S. Coulon, and R. P. Fuchs, “Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli.,” Proc. Natl. Acad. Sci. U. S. A., 108, 19311–6, (2011).
- R. Hirmondó et al., “Cross-species inhibition of dUTPase via the Staphylococcal Stl protein perturbs dNTP pool and colony formation in Mycobacterium,” DNA Repair (Amst)., 30, 21–27 (2015).
- Wilson, P. M. et al. “A novel fluorescence-based assay for the rapid detection and quantification of cellular deoxyribonucleoside triphosphates”. Nucleic Acids Res. 39, 1–15 (2011).
- K. Kino and H. Sugiyama, “Possible cause of G-C-->C-G transversion mutation by guanine oxidation product, imidazolone.,” Chem. Biol., 8, 369–78 (2001).
