|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. Hirsch Edit
Development of real-time process monitoring and control system for the high-quality production of biologics
Introducing the research area
In the last decade, the demand for biotechnology-derived therapeutics, especially monoclonal antibodies, has increased significantly in the pharmaceutical market [1]. However, due to the extremely large size and complex structure of these protein-type drugs, their consistent quality, efficient and safe production poses several challenges for pharmaceutical manufacturers.
The manufacturing process of mAbs consists of several successive complex steps that can be divided into three major parts: protein production using cellular systems (upstream); purification of the produced protein (downstream); and converting the purified drug substance into a drug product (formulation). The biggest challenge during production is that the quality of the product can be affected by all process parameters. Therefore, it is essential to ensure properly monitored and strictly regulated conditions throughout the production process, which requires the development of new innovative technologies.
Brief introduction of the research place
My research is carried out in a FirePharma research group under the supervision of Dr. György Marosi and Dr. Zsombor Nagy. The group focuses on developing innovative, automated, and highly regulated technologies that can serve as a basis for efficient, safe, and high-quality pharmaceutical production in the future.
History and context of the research
Monoclonal antibodies are much larger in size and have greater structural complexity than conventional chemically synthesizable drugs, thus they can only be produced by the cells of a living organism [2]. It is essential to ensure appropriate cultivation conditions during production, as these living organisms are very sensitive to the environmental changes that surround them [3]. Even a small modification of process parameters can have a large impact on both cell growth and viability, as well as on the produced monoclonal antibodies. Therefore, a better understanding of the effect of each culture parameter on the produced protein is crucial for achieving the right amount of high-quality product.
Since the launch of the Process Analytical Technology (PAT) initiative in 2004, inline and online analytical methods are preferred by the pharmaceutical industry to measure critical parameters because of their ability to provide valuable information quickly, non-destructively, and in real-time without sampling [4]. In the bioreactor cell culture process, inline/online sensors are already widely used for measuring classical physicochemical parameters (e.g., pH, temperature, or dissolved oxygen) [5]. On the other hand, there are several other critical parameters, such as nutrient and by-product concentration, viable cell density, or protein concentration, whose inline analysis is currently not sufficiently addressed in the industry, thus they are determined by daily sampling and analytical tests separated in time and/or space (atline and offline). However, for both offline and atline measurements, the delay between sample removal and the availability of results prevents real-time decision-making and immediate feedback to the process, thereby increasing the chances of producing a poor-quality product or scrap.
The research goals, open questions
My research aims to develop new and innovative technological solutions for the bioreactor cultivation of monoclonal antibody-producing mammalian cells, with particular emphasis on the practical application of PAT principles as well as advanced control strategies. Inline Raman spectroscopy, as a promising PAT tool is applied, as it allows the simultaneous measurement of several different critical process parameters during cell culture. My work focuses on investigating the concentration of nutrients essential for cells (glucose and amino acids) as well as harmful by-products (lactate and ammonia) formed during cell metabolism.
Using the developed Raman spectroscopy-based monitoring and control system, I would like to gain a deeper understanding of the complex and dynamically changing biological processes that take place during culture. This provides an opportunity to develop feeding strategies where the nutrients are replenished based on the requirements of the cells using an automated system. In my research, the goal is to maintain the concentration of critical nutrient and by-product components within the optimal range, thereby providing more favorable culture conditions for cells and enabling robust and reproducible production of high-quality monoclonal antibodies.
Methods
In my research, I deal with bioreactor cultivation of adalimumab-producing Chinese Hamster Ovary (CHO) cell line. Initially, the cell culture is maintained in a small-scale shake flasks system using incubators (36.5° C and 5% CO2), subsequently in a large-scale (2 liter) glass bioreactor. The bioreactor is equipped with the following inline probes to monitor critical culture parameters: thermometer, pH probe, dissolved oxygen probe, dielectric spectrometer (viable cell count sensor), and Raman spectrometer immersion probe (Figure 1).
During the culture process, the nutrients required for cell growth are replenished by multicomponent feed solutions (Feed A and Feed B) and a glucose stock solution. The reactor is sampled 1–2 times per day, during which the number of living and dead cells and the viability are examined atline by microscopy. After freezing the sample, the various nutrient and by-product concentrations (glucose, amino acids, lactate, and ammonia) are determined by offline measurement with a Cedex bioanalyzer and a Skyam automated amino acid analyzer.


Figure 1. Bioreactor culture of mammalian cells using inline Raman spectroscopy
Raman spectroscopy is a vibrational spectroscopic method based on the inelastic scattering of the monochromatic light beam. The Raman spectra is a kind of fingerprint, where each peak or band corresponds to a given vibration of the molecule. The great advantage of Raman measurement is that it can provide both qualitative and quantitative information about the sample. For quantitative analysis of critical parameters in cell culture, multivariate data analysis (MVDA) techniques have to be applied to extract relevant information from the Raman spectra. In the field of biotechnology, the method of Partial Least Squares (PLS) regression method is one of the most commonly used [6]. Before the Raman spectra evaluation, various mathematical preprocessing procedures (baseline correction, normalization, and centering) need to be performed to reduce or eliminate interfering effects, such as the fluorescence activity of biological molecules or the occurrence of solid particles and gas bubbles.
Results
During my research, Raman-based calibration models were developed using the PLS regression method for in-line monitoring of nutrient and by-product concentrations (glucose, lactate, and amino acid) during bioreactor CHO cell cultivations. For the robustness of the analytical method, it was important to collect a large dataset that well describes the biological variability of the system. Figure 2 illustrates the PLS model development process, in which data from nine shake flasks and seven bioreactor culture experiments were used. This resulted in a total of 204 Raman spectra and the corresponding offline concentration (glucose, lactate, amino acids) data pairs.
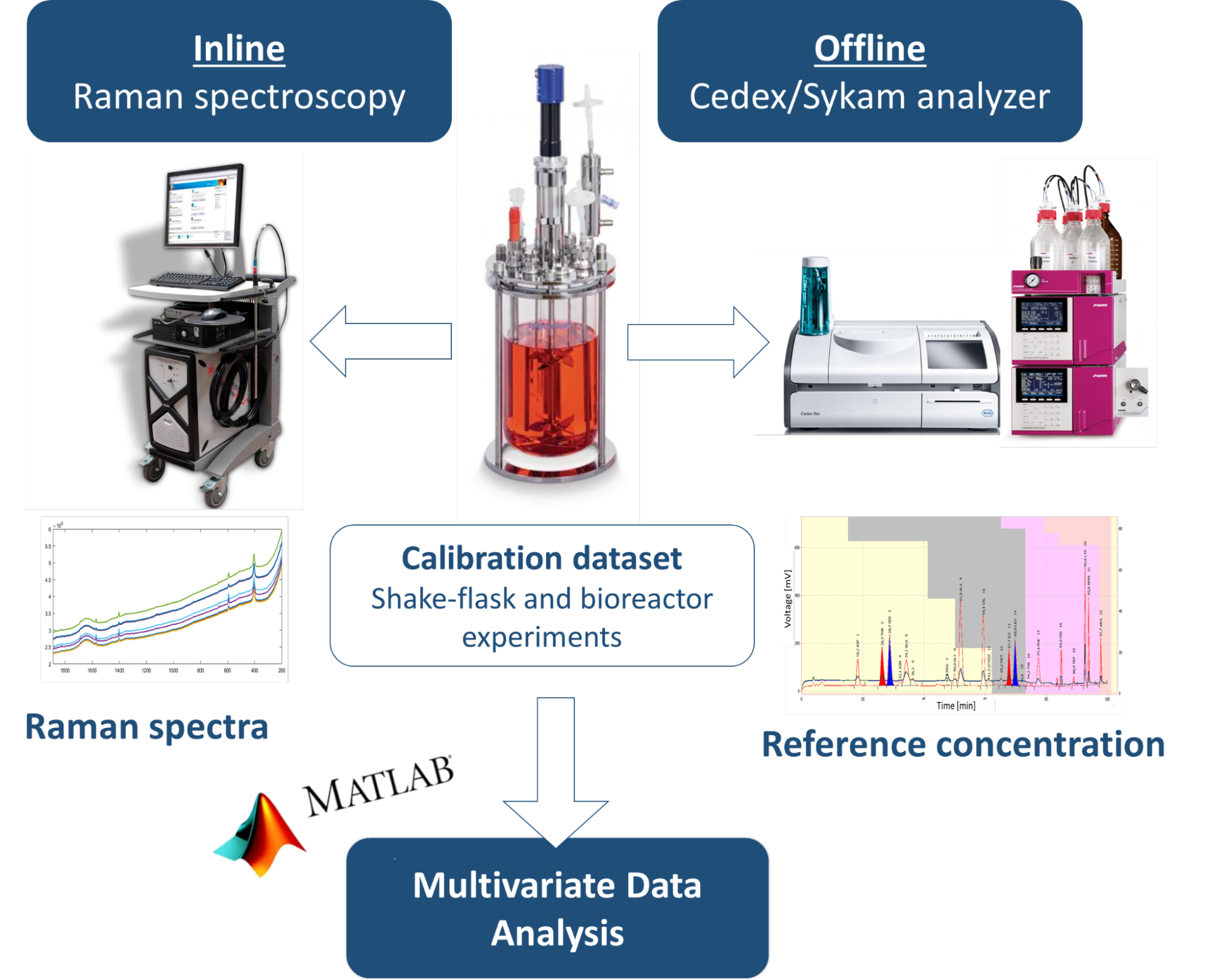
Figure 2. Calibration model development process using the PLS method
Robust and accurate PLS calibration models were built for 16 individual amino acids in addition to glucose and lactate. Figure 3 shows the correlation between the measured and predicted concentration values measured by the Raman-based glucose model. To investigate the predictive performances of the models, an independent bioreactor cultivation experiment was used as a validation run, during which the concentration change of the individual components could be monitored in real time with Raman measurement (Figure 3).
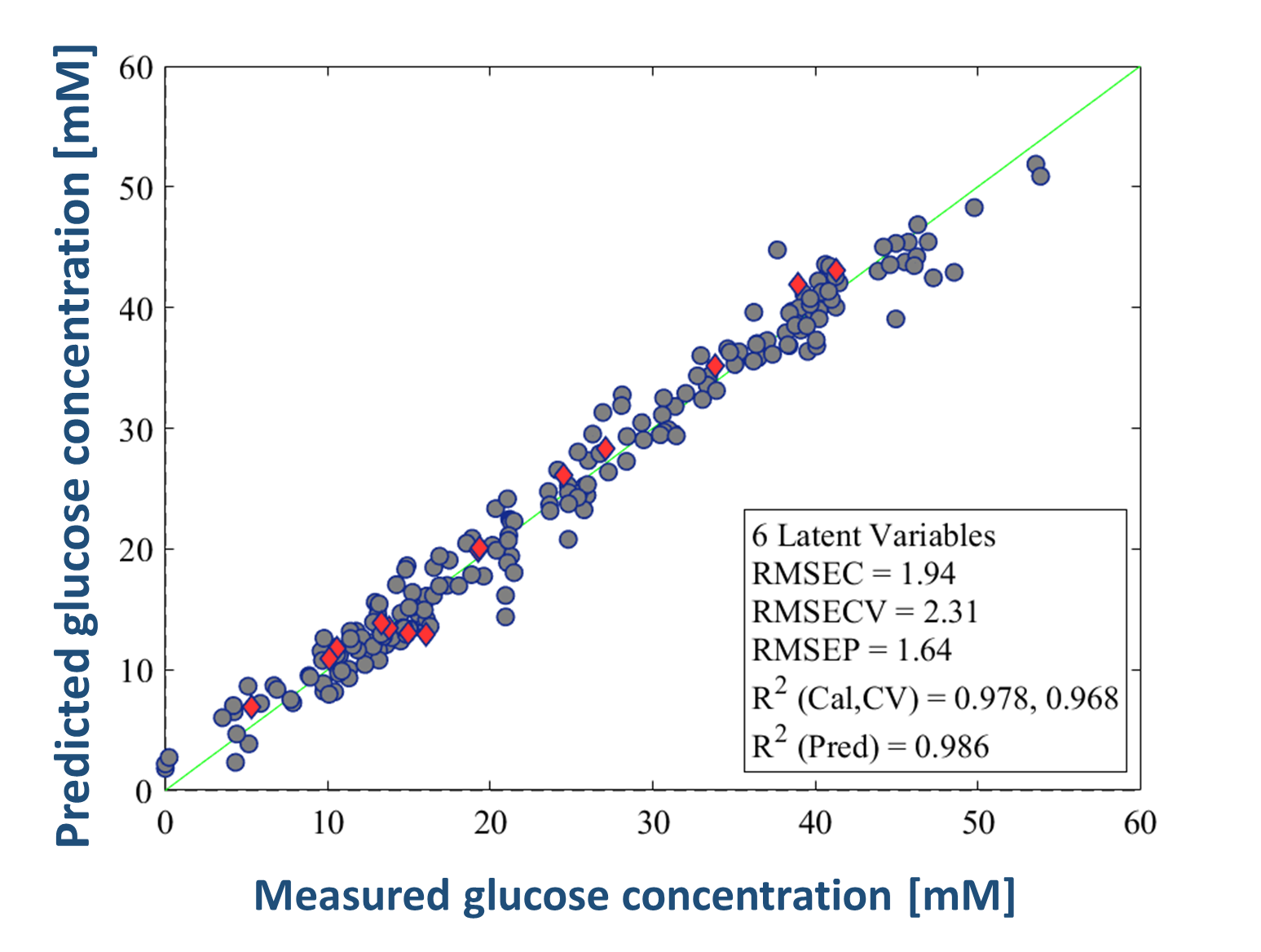
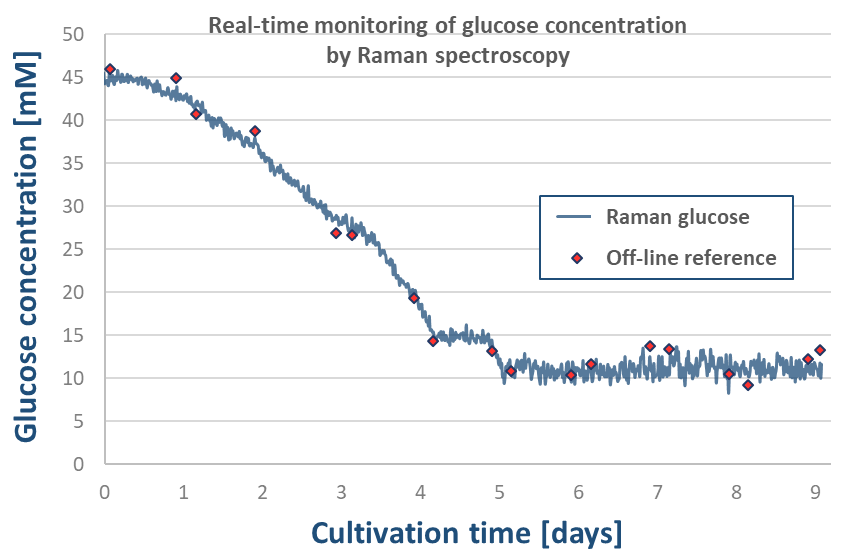
Figure 3. Glucose-based PLS calibration model (left), model validation by real-time Raman monitoring during an independent bioreactor experiment (right)
Subsequently, dynamic feeding strategies were implemented by the developed Raman-based monitoring and control system, where the goal was to maintain the nutrients at a constant level by controlling the addition of concentrated glucose solution and multicomponent feed solutions. The feedings were performed according to the glucose (glucose solution) and arginine (feed solutions) concentrations monitored in real-time using an automatic pump delivery system (Figure 4).
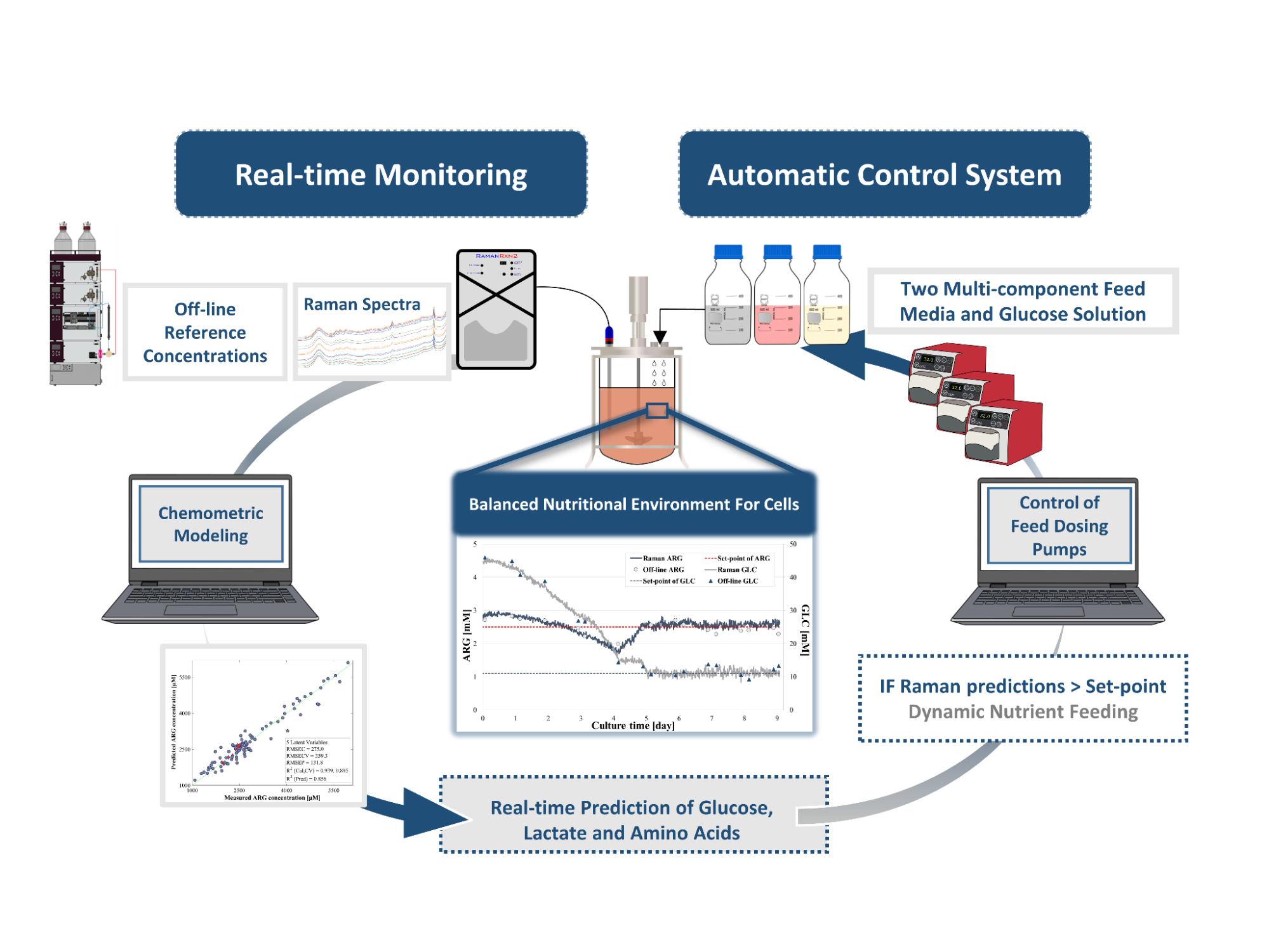
Figure 4. Self-developed, automated monitoring and control system for efficient feeding strategies based on the requirements of the cells
Using the Raman-based monitoring and control system, more favorable culture conditions were maintained during the process. As a result, a culture with higher cell concentrations and sustainable for a longer period, as well as higher antibody production could be achieved.
Expected impact and further research
The Raman spectroscopy-based monitoring and control system developed during my research provides the opportunity to measure those critical parameters of the mammalian cell culture process in real-time which are currently determined in the industry by sampling and offline/atline analytical methods. The future application of the system can provide the opportunity to ensure optimal cell culture conditions and to produce the appropriate amount of the desired quality product more easily and economically. In addition, the results presented here can serve as guidance for the pharmaceutical industry to integrate automated process monitoring and control systems based on real-time measurement, which are increasingly required by the authorities.
A significant part of the research work was carried out in the frame of the FIEK_16-1-2016-0007 project in collaboration with Richter Gedeon Plc. The collaboration is an excellent indicator of the significant industrial interest in the topic, and the results may be exploited in the near future.
Publications, references, links
List of corresponding own publications
[I] Hirsch E, Pataki H, Domján J, Farkas A, Vass P, Fehér C, Barta Z, Nagy ZK, Marosi GJ, Csontos I. Inline noninvasive Raman monitoring and feedback control of glucose concentration during ethanol fermentation. Biotechnol Prog. 2019 Sep;35(5):e2848.
IF: 2.334 C: 15
[II] Domján J, Fricska A, Madarász L, Gyürkés M, Köte Á, Farkas A, Vass P, Fehér C, Horváth B, Könczöl K, Pataki H, Nagy ZK, Marosi GJ, Hirsch E. Raman-based dynamic feeding strategies using real-time glucose concentration monitoring system during adalimumab producing CHO cell cultivation. Biotechnol Prog. 2020 Nov;36(6):e3052.
IF: 2.513 C: 6
[III] Domján J, Vass P, Hirsch E, Szabó E, Pantea E, Andersen SK, Vigh T, Verreck G, Marosi G, Nagy ZK. Monoclonal antibody formulation manufactured by high-speed electrospinning. Int J Pharm. 2020 Dec 15;591:120042.
IF: 5.875 C: 6
[IV] Vass P, Pantea E, Domokos A, Hirsch E, Domján J, Németh Á, Molnár M, Fehér C, Andersen SK, Vigh T, Verreck G, Csontos I, Marosi G, Nagy ZK. Electrospun Solid Formulation of Anaerobic Gut Microbiome Bacteria. AAPS PharmSciTech. 2020 Jul 31;21(6):214.
IF: 3.246 C: 4
[V] Hirsch E, Pantea E, Vass P, Domján J, Molnár M, Suhajda Á, et al. Probiotic bacteria stabilized in orally dissolving nanofibers prepared by high-speed electrospinning. Food Bioprod Process. 2021;128:84–94.
IF: 4.32 C: 3
[VI] Domján J, Pantea E, Gyürkés M, Madarász L, Kozák D, Farkas A, Horváth B, Benkő Z, Nagy ZK, Marosi G, Hirsch E. Real-time amino acid, and glucose monitoring system for the automatic control of nutrient feeding in CHO cell culture using Raman spectroscopy. Biotechnol J. 2022 May;17(5):e2100395.
IF: 4.677 C: 0
Table of links.
List of references.
