|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. FARKAS Attila
Application of Image Analysis for Real-time Monitoring and Control of Pharmaceutical Processes
Introducing the research area
The pharmaceutical industry is currently undergoing a significant transformation to develop more efficient processes and consistent product quality. This involves both how pharmaceuticals are manufactured, as well as how their quality is controlled. Novel continuous drug manufacturing processes offer several advantages over traditional batch technologies1. In-line quality control (QC) plays a critical role in realizing these benefits, as it enables manufacturers to monitor, optimize, and control manufacturing processes closely2. Image analysis techniques, with their ability to provide detailed information about particle size, shape, and morphology, have great potential in pharmaceutical applications for real-time QC. Hence, my research explores the feasibility of in-line image analysis for real-time process monitoring and control of various pharmaceutical processes, intending to enhance process optimization and establish advanced quality control methods.
Brief introduction of the research place
FirePharma research group is committed to advancing practical solutions in the field of pharmaceutical manufacturing and real-time QC techniques. Our focus lies in developing innovative methodologies to address the current challenges facing pharmaceutical manufacturers. By collaborating with both Hungarian and international pharmaceutical companies, we ensure that our research outcomes are relevant to industry needs.
History and context of the research
In modern pharmaceutical manufacturing, continuous process monitoring, and control is essential to ensure product quality and meet regulatory requirements3. Process Analytical Technology (PAT) sensors play a crucial role in enabling continuous QC, as they can provide real-time data about the critical quality attributes (CQAs) (Figure 1).
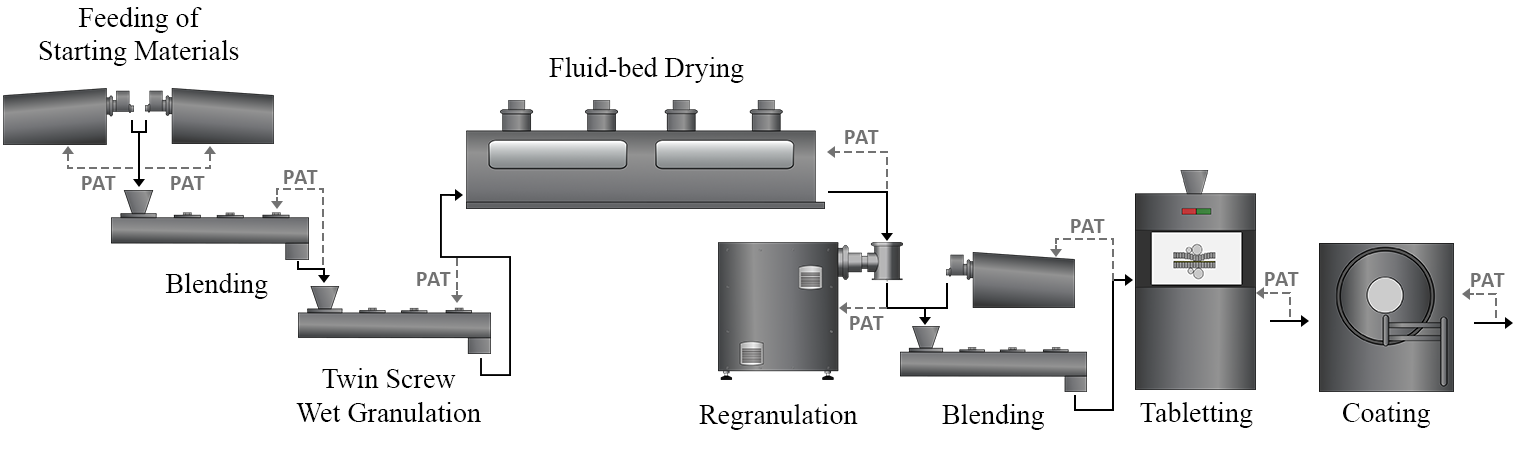
Figure 1. Schematic of a continuous tablet manufacturing line with potential uses of PAT sensors.
Reliable PAT sensors are vital for effective real-time QC, allowing manufacturers to closely monitor manufacturing processes, detect deviations in real time, and take corrective actions accordingly. This ensures that the product quality remains within the specified limits and reduces the need for end-product testing, resulting in cost savings and improved overall process efficiency.
Particle size is a CQA that directly impacts material processability and end-product properties, such as dissolution4. Consequently, there is a great need for in-line particle size measurement. In recent years, in-line imaging has emerged as a promising multi-purpose PAT tool capable of providing real-time information regarding particle size, shape, morphology, coating thickness, etc.
The research goals, open questions
As mentioned earlier, there is a strong need for in-line PAT sensors in the pharmaceutical industry to facilitate effective real-time quality control. Consequently, the main objectives of my research can be summarized as follows:
1. Investigate the applicability of an imaging system for real-time particle size measurement and control during twin-screw wet granulation (TSWG).
2. Overcome the difficulties of particle size measurement during high mass flow applications and apply in-line image analysis in an interconnected continuous granule production line to study the effect of several critical process parameters on the resulting granule size in real time.
3. Investigate the applicability of real-time image analysis for camera-based mass flow measurement and micro-dosing with simultaneous particle size measurement.
4. Further develop image analysis-based particle size measurement by utilizing state-of-the-art, artificial intelligence (AI)-based image processing algorithms to overcome the challenge of analyzing overlapping particles and extend the in-line applicability of image analysis for pharmaceutical processes, including batch processes.
Methods
1. Monitoring and control of TSWG
A custom real-time imaging system consisting of a process camera, light source, PC, and image analysis software was developed. The placebo blend of 80:20 lactose:starch mixture was granulated with a liquid produced by dissolving PVPK30 in abs. ethanol. The camera was mounted onto a twin-screw granulator to measure the particle size of granules produced with varying liquid-to-solid (L/S) ratios. A custom controller facilitated communication between the software and the pump for feedback control. During the feedback control experiments, the system regulated the liquid addition rate based on real-time particle size measurement to maintain the desired size.
2. Monitoring of an interconnected granule production line
An interconnected continuous granule production line was also investigated with in-line imaging (Figure 2). The developed camera-based particle sizing system was installed directly after the mill for the in-line particle size measurement of the regranulated product. A vibratory feeder was used to temporarily reduce the particle flow to avoid the overlapping of granules in the high mass-flow stream (~800g/h). The system was then used to demonstrate the applicability of a modern PAT tool for rapid mapping of the relations between several Critical Process Parameters (CPP) and the resulting CQAs. The experiments involved the investigation of several CPPs: L/S ratio, milling tool type, sieve hole diameter, and milling intensity.
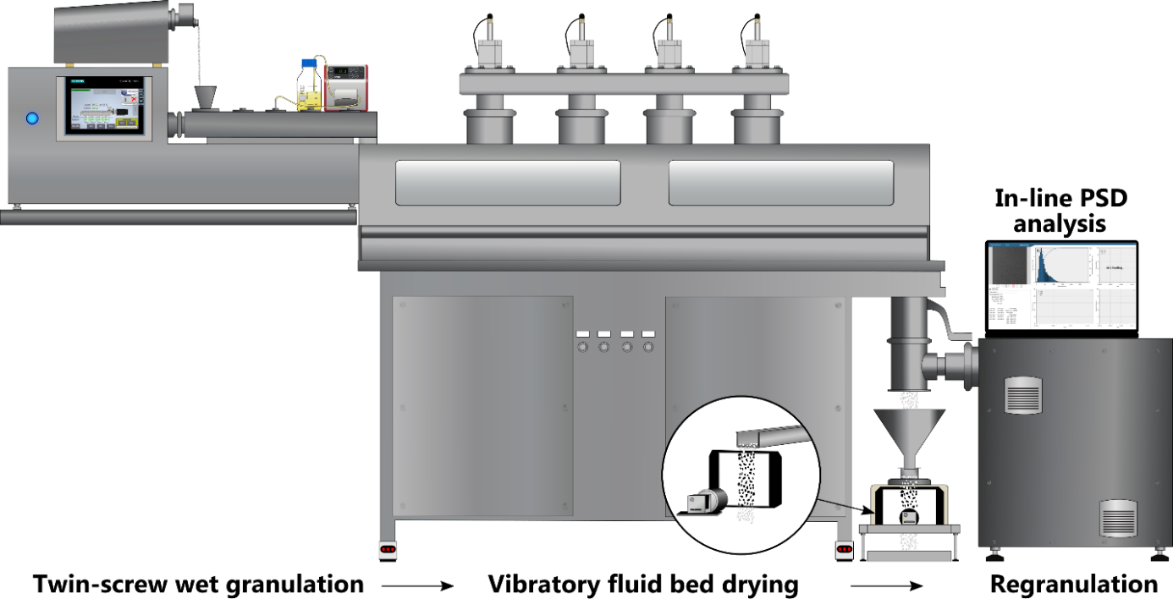
Figure 2. Physical arrangement of the Continuous Granule Manufacturing line.
3. Camera-based (videometric) mass measurement
The feasibility of videometric powder micro-dosing and ultra-low powder mass flow measurement was also investigated. During the experiments, caffeine was fed via a single-screw feeder, while an analytical scale was used for reference mass measurement. The videometric system was used for the micro-dosing of caffeine with different weight targets (25–500 mg). The system was then used for mass flow measurement in the range of 0.25–1 g/min. After tuning a PID controller, the system was used in feedback control mode. During these experiments, the feeder speed was automatically regulated by the image analysis software based on the mass flow measured with the videometric system in order to keep the powder mass flow to the predefined value.
4. AI-based image analysis for real-time particle size measurement
The feasibility of AI-based real-time particle size measurement was also investigated using a process endoscope (Figure 3.).
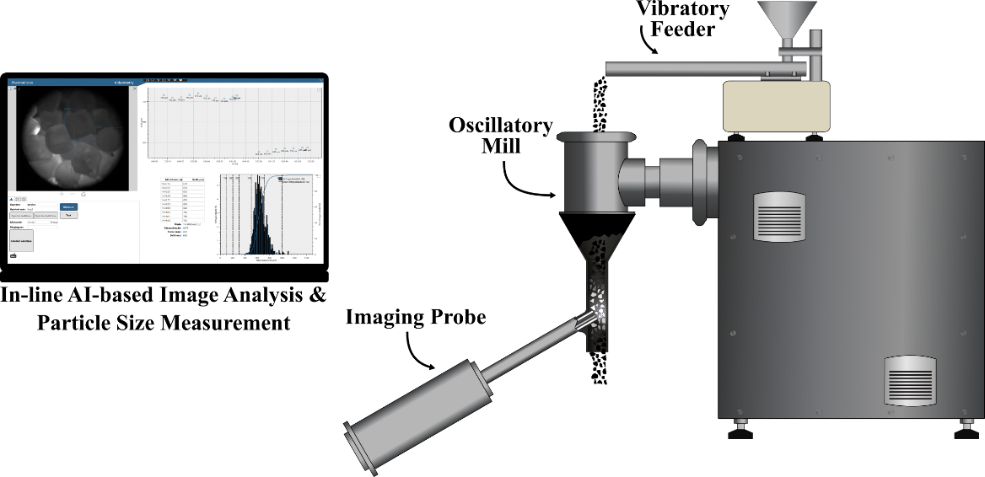
Figure 3. Schematic of the AI-based in-line imaging system.
A dataset was created to train the AI by manually fitting a polygon on the outline of NaCl particles in 300 images. The resulting AI model was used to measure the particle size of different NaCl samples. The system was then installed into a continuous mill for in-line particle size measurement, where a 3D-printed funnel guided the milled particles in front of the probe. During the experiment, NaCl (particle size: 710–1000 µm) was milled using a screen with an 800 µm hole diameter.
Results
1. Monitoring and control of TSWG
The in-line system precisely detected the monotonically increasing function between the L/S ratio and the granule particle size, indicating the accurate behavior of the developed PAT tool. The system was then used in feedback control mode, where the image analysis software automatically regulated the liquid addition rate based on the measured particle size. Figure 4 shows the behavior of the controller during system startup.
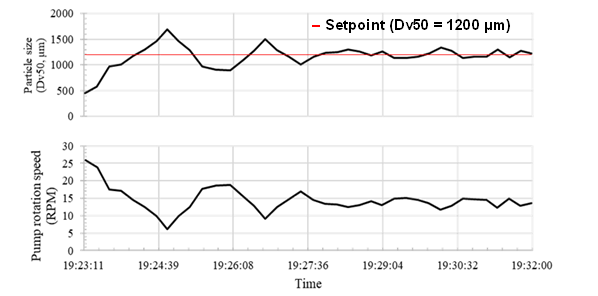
Figure 4. Particle size determined with in-line particle size analysis and the feeding rate of the peristaltic pump.
Thanks to the automatic regulation of the liquid feed rate, the initial small particle size was compensated for with a decreasing oscillation of both the particle size and the pump speed over time.
2. Monitoring of an interconnected granule production line
The vibratory feeder allowed for in-line particle size measurement in the granule production line by temporarily lowering the mass flow of the product. Using the developed PAT tool, the relations between the CQA (particle size) and various CPPs (milling tool type, screen hole diameter, milling intensity, L/S ratio) were efficiently mapped. As expected, higher milling intensity and smaller screen hole diameter resulted in reduced product particle size. Figure 5 shows that the image analysis-based tool was able to verify these presumptions.
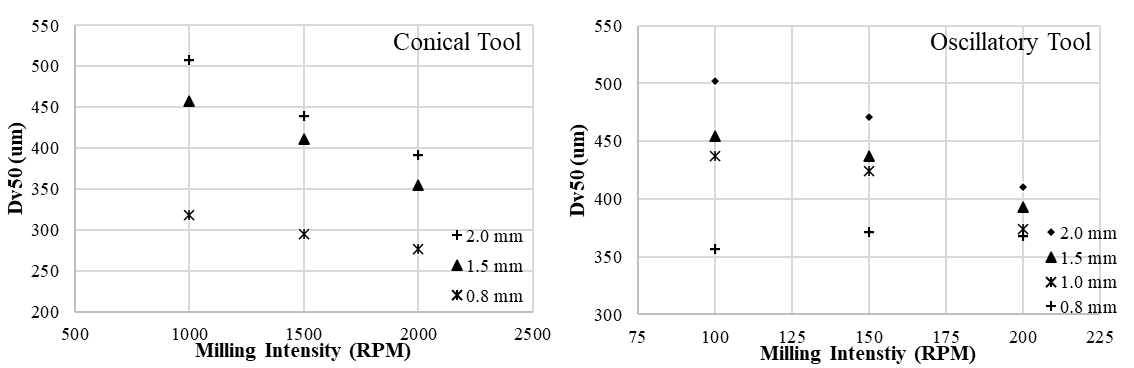
Figure 5. Camera-based Dv50 values obtained with different milling settings.
In the experiments focusing on granulation, the developed system was also able to detect an increase in the particle size of the regranulated product with an increase in L/S ratios.
3. Camera-based mass measurement
The videometric system accurately dosed different target weights (25–500 mg) with a relative prediction error below 5% (using a microbalance as reference), demonstrating its precision for powder micro-dosing. Next, the developed system successfully measured ultra-low powder mass flows (<1g/min) with less than 5% prediction error as well.
Lastly, the system was used in feedback control mode. Step disturbances were applied to the system to test the controller’s performance. The controller was able to quickly react to the disturbance through the automatic control of the feeder speed, modifying the mass flow to the desired value (Figure 6).
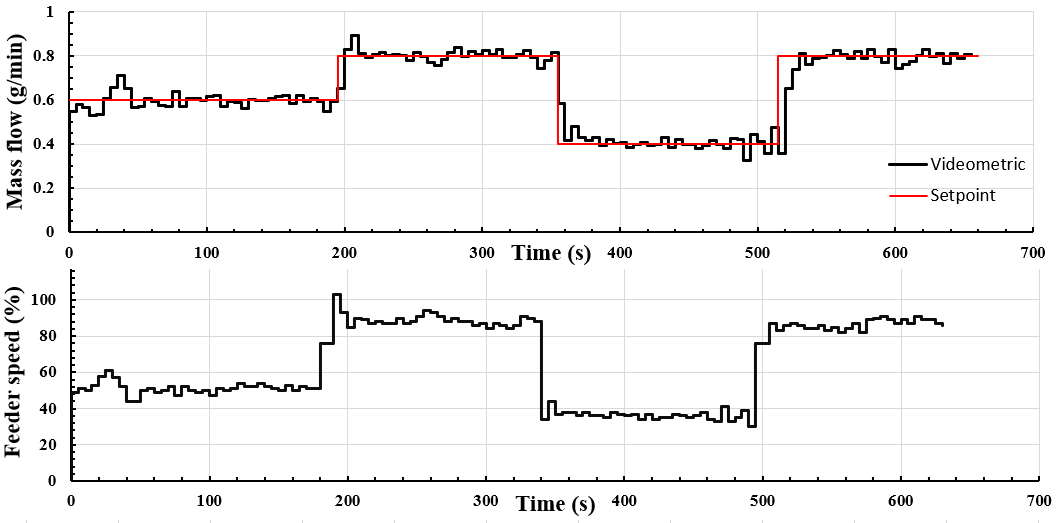
Figure 6. Videometric mass flow measurement and the regulated feeder speed.
The videometric system was also used for simultaneous in-line particle size measurement, which provided particle size results very similar to off-line reference results, thus serving as a multi-purpose PAT tool.
4. AI-based image analysis for real-time particle size measurement
Contrary to conventional image analysis algorithms, the AI model could identify overlapping crystals. The model was used to measure the particle size of different NaCl samples, where the results obtained with the imaging system showed excellent correlation with reference results.
Finally, the imaging system was fitted into a continuous mill for in-line particle size measurement. The AI-based method effectively detected the particle size reduction as a result of milling and yielded a Dv50 value similar to the reference results obtained with Laser Diffraction (LD). The AI-based tool proved to be a promising solution for real-time pharmaceutical particle size measurement.
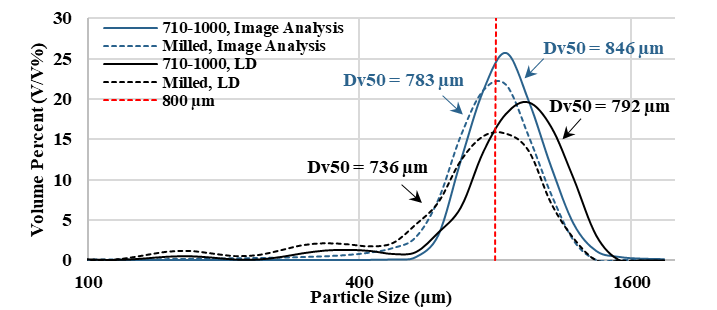
Figure 7. Measured PSDs with LD (dashed lines) and Image Analysis (continuous lines) from the milling experiment (blue) and the starting material (black).
Expected impact and further research
The developed real-time imaging tool can serve as a versatile tool to gain invaluable insights into pharmaceutical processes, both during process development and manufacturing. The system is useful for the academic, as well as the industrial sector, as it can be efficiently used to monitor numerous CQAs, e.g., particle size in real time. The real-time obtained data facilitates a deeper understanding of the process during process development, while during manufacturing, this data helps to steer the process in the right direction in case of any disturbances, ultimately ensuring the highest product quality.
Publications, references, links
List of corresponding own publications
[2] L. Madarász, Á. Köte, B. Hambalkó, K. Csorba, V. Kovács, L. Lengyel, G. Marosi, A. Farkas, Zs. K. Nagy, A. Domokos: In-line particle size measurement based on image analysis in a fully continuous granule manufacturing line for rapid process understanding and development, Int. J. Pharm, 612, (2022), 121280, IF: 6.510, D1
[3] L. Madarász, Á. Köte, M. Gyürkés, A. Farkas, B. Hambalkó, H. Pataki, G. Fülöp, G. Marosi, L. Lengyel, T. Casian, K. Csorba, Zs. K. Nagy, Videometric mass flow control: A new method for real-time measurement and feedback control of powder micro-feeding based on image analysis, Int. J. Pharm, 580, (2020), 119223, IF: 5.875, D1
[4] L. Madarász, L. A. Mészáros, Á. Köte, A. Farkas, Zs. K. Nagy, AI-based Analysis of In-line Process Endoscope images for Real-time Particle Size Measurement in a Continuous Pharmaceutical Milling Process, International Journal of Pharmaceutics, Volume 641, 25 June 2023, 123060, IF: 6.51, D1
[5] A. Domokos, L. Madarász, Gy. Stoffan, K. Tacsi, D. Galata, K. Csorba, P. Vass, Zs. K. Nagy, H. Pataki, Real-Time Monitoring of Continuous Pharmaceutical Mixed Suspension Mixed Product Removal Crystallization Using Image Analysis, Org Process Res Dev, 26 (2021), 149–158., IF: 3.858, Q1
[6] D. Farkas, L. Madarász, Zs. K. Nagy, I. Antal, N. Kállai-Szabó, Image analysis: A versatile tool in the manufacturing and quality control of pharmaceutical dosage forms, Pharmaceutics, 13 (2021), 685., IF: 6.525, Q1
[7] A. Domokos, É. Pusztai, L. Madarász, B. Nagy, M. Gyürkés, A. Farkas, G. Fülöp, T. Casian, B. Szilágyi, Zs. K. Nagy, Combination of PAT and mechanistic modeling tools in a fully continuous powder to granule line: Rapid and deep process understanding, Powder Technology, Volume 388, 70–81, 26 April, 2021. IF: 5.64, Q1
Table of links.
[Process Analytical Technology]
List of references.
1S. Chatterjee, FDA Perspective on Continuous Manufacturing, IFPAC Annual Meeting Baltimore, 2012.
2Food and Drug Administration, 2019. Quality Considerations for Continuous Manufacturing; Guidance for Industry
3International Council for Harmonization, 2023. Q13 Continuous manufacturing of drug substances and drug products
4Wünsch, J.H. Finke, E. John, M. Juhnke, A. Kwade, The influence of particle size on the application of compression and compaction models for tableting, International Journal of Pharmaceutics, 599 (2021) 120424.
