|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Applied Biotechnology and Food Science
Supervisor: Dr. FEHÉR Csaba
Complex utilization of agricultural by-products using bacterial and nonconventional yeast-derived enzymes in consolidated bioprocesses
Introducing the research area
Lignocellulose-based biomass is currently the most abundant raw material, and processing has not been fully resolved/exploited yet. Biorefining can offer a solution to this challenge. By hydrolyzing the carbohydrate content of lignocellulosic raw materials, a variety of value-added components can be produced, both through chemical synthesis and fermentation processes. The biorefinery concept offers a promising alternative for the production of xylitol or other products, where the enzymatic and microbial breakdown of lignocellulose-based biomass followed by the conversion of the degradation products sugars into valuable compounds can be implemented in the same space – it is also called consolidated bioprocessing. While it is known that many nonconventional yeasts possess good product-forming enzyme systems (e.g., xylose reductase), their hydrolytic enzyme formation remains a less explored area. Therefore, the main objective of my doctoral work is to search for nonconventional yeasts that exhibit hydrolytic enzyme activity (for lignocellulose) and are also capable of producing some valuable products (e.g., xylitol, organic acids, etc.) through consolidated bioprocessing.
Brief introduction of the research place
I am conducting my research at the Department of Applied Biotechnology and Food Science, in the Biorefinery Research Group, under the supervision of Csaba Fehér. Our group has been dedicated to the valorization of lignocellulosic by-products, which is relevant today as sustainability and circular economy principles are gaining more importance.
History and context of the research
The utilization of lignocellulosic by-products, which are renewable and cost-effective raw material sources, holds significant economic and social benefits [1]. However, the widespread adoption of industrial processes targeting lignocellulosic biomass utilization is hindered by the high cost of enzymes and the complex, multi-step technology required, resulting in substantial investment and operational expenses [2]. Through the integration of numerous steps and efficient enzyme production, integrated bioprocessing could offer a cost-effective solution [3].
Figure 1. The biorefinery concept.
The thorough investigation of new microorganisms (both bacteria and yeasts) required for this purpose, not only serves the advancement of relevant scientific fields such as yeast and bacterial microbiology, fermentation, biorefining, and industrial biotechnology but also contributes to the realization of environmentally and economically sustainable industrial processes (biorefineries) capable of producing high-value bioproducts from lignocellulosic raw materials. These integrated biorefining processes are essential for achieving a circular bio-based economy, thus, their research and development hold outstanding economic, environmental, and social significance. My doctoral research offers the potential to achieve numerous new scientific results: the examination and production of hydrolytic enzymes by nonconventional yeasts and bacteria, investigation of their product-forming properties, applicability of monocultures and/or mixed cultures in consolidated bioprocessing, and enhancement of bioprocessing efficiency through parameter optimization.
The research goals, open questions
The main objective of my research is the degradation of lignocellulosic agricultural by-products (e.g., wheat bran, brewer's spent grain, waste paper) followed by their conversion into valuable products (e.g., xylitol, ethanol) through integrated bioprocessing using bacterial and/or nonconventional yeast-derived enzymes.
The main aims of my research are as follows:
- Investigation of new bacterial and nonconventional yeast strains in terms of hydrolytic enzyme activity and product formation.
- Determination of optimal conditions for enzyme activity measurement and enzyme production in terms of hydrolytic enzyme-producing strains.
- Identification of products produced by product-forming strains and optimization of their production conditions (with a particular focus on xylitol).
- Examination of strains with good hydrolytic enzyme production and product-forming properties in monoculture and/or mixed culture populations.
- Implementation of consolidated bioprocessing using shake flasks initially and later in laboratory-scale fermenters, using monoculture or mixed culture populations.
Methods
Investigation of xylanase production by Cellulomonas phragmiteti
Enzyme production was examined under aerobic conditions in submerged cultures using various lignocellulosic by-products (waste paper, wheat bran, brewer's spent grain, rye bran, rice straw, and DDGS). Xylanase activity was measured using the DNS method. Besides xylanase, other activities were also measured: CMC-ase, β-xylosidase, β-glucosidase, arabinofuranosidase, and cellobiohydrolase. CMC-ase activity was also measured using a DNS method, while other activities were measured based on p-nitrophenol release. The pH and temperature optima of the xylanase activity produced by C. phragmiteti on waste paper were investigated. Regarding pH and temperature, the experiments were conducted within a pH range of 3–11, and a temperature range of 25–85 °C, respectively. Enzyme stability tests were also conducted at 45 °C and 55 °C.
Investigation of hydrolytic enzyme production by nonconventional yeasts
Komagataella pseudopastoris, Spencermartinsiella sp., Spencermartinsiella ligniputrida, Spencermartinsiella europaea, Sugiyamaella novakii, Kuraishia molischiana, Candida maritima, Candida hawaiinensis, Scheffersomyces stipitis, Candida allociferri, Ogatae pilisensis, Geotrichum klebahnii, and Candida baotianensis have been investigated by us. First of all, Congo Red staining was performed on xylan-supplemented agar plates. Strains showing clear zones were involved in further experiments. They were cultured in xylan model substrates under aerobic conditions in submerged cultures. Xylanase activity was measured from the fermentation broth using the DNS method, and side activities mentioned for C. phragmiteti are also planned to be investigated. In the cases where xylanase activity is observed, we aim to optimize the conditions for activity measurement and enzyme production.
Investigation of valuable products produced by nonconventional yeasts
The strains mentioned in the previous section are cultivated on various carbon sources (glucose, xylose, arabinose, etc.) under different conditions (aerobic, microaerobic, anaerobic). Samples are taken daily to monitor cell growth, and the cell-free supernatants are prepared for HPLC analysis to follow carbon source consumption and identify potential products.
Optimization of xylitol production by Candida baotianensis
The optimal conditions for xylitol production by C. baotianensis were investigated within shake flask experiments (aeration, medium composition, temperature, pH). Through experimental design (full factorial, orthogonal experimental design (32)), the filling level of the flask and the initial xylose concentration were examined to reveal how they can influence xylitol yield and volumetric productivity.
Results
Characterization of the xylanase produced by Cellulomonas phragmiteti
C. phragmiteti was cultivated in LB (Luria-Bertani), MM (Minimal), and King’s B media supplemented with different lignocellulosic residues as carbon sources. The LB medium supplemented with the waste paper was found the most suitable for xylanase production by C. phragmiteti.
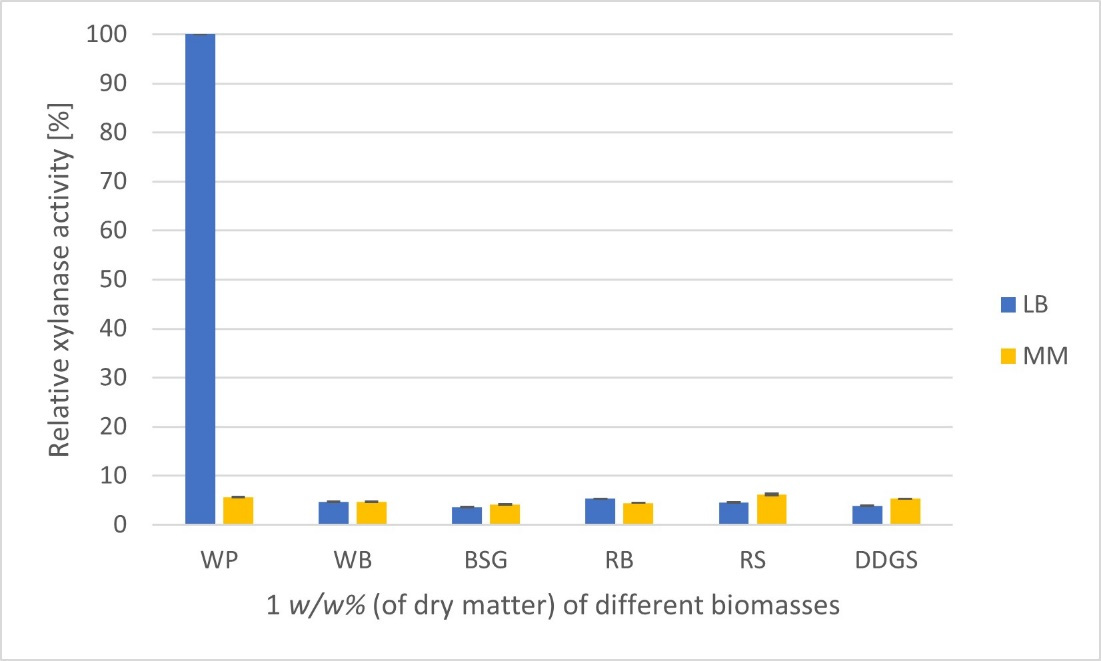
Figure 2. Relative xylanase activities [%] produced by C. phragmiteti on different carbon sources (1 w/w% of dry matter) in LB and MM media. WP—waste paper; WB—wheat bran; BSG—brewer’s spent grain; RB—rye bran; RS—rice straw; DDGS—distillers dried grains with solubles.
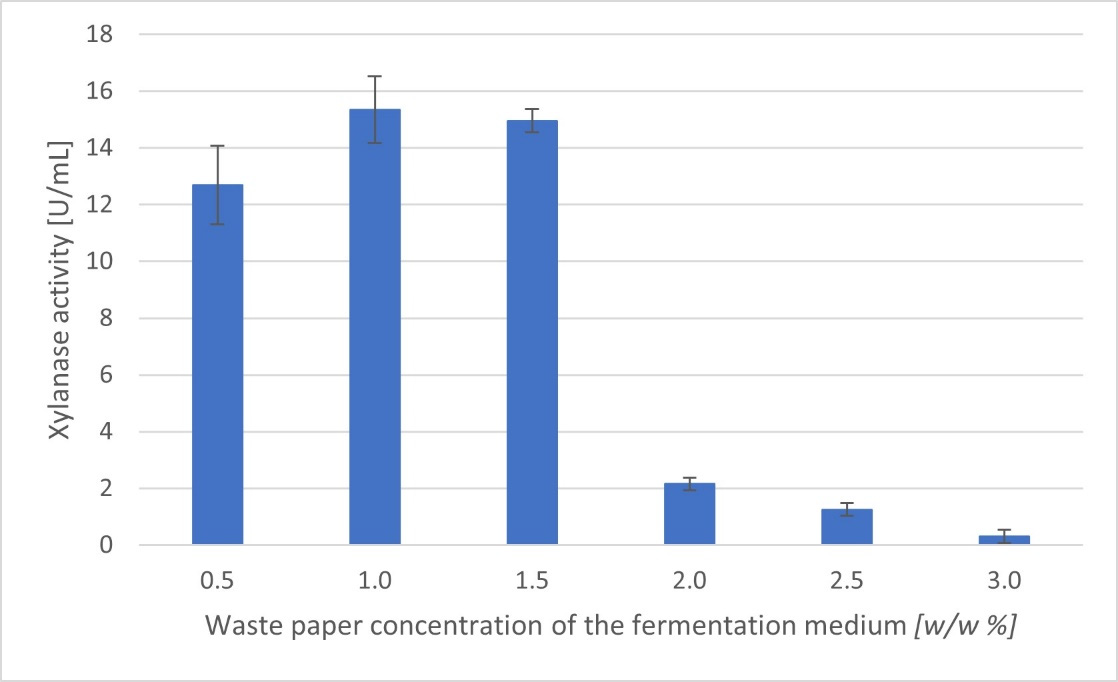
It was also investigated how different concentrations of waste paper can affect the enzyme production. The highest xylanase activity (15.3 U/mL) was achieved by using 1 w/w% waste paper.
Figure 3. Xylanase activities [U/mL] obtained in LB medium containing different amounts of waste paper (w/w% of dry matter).
Side activities were also measured from cell-free supernatants. β-xylosidase activity was the highest (0.61 U/mL). Since C. phragmiteti has been characterized as a salt-tolerant bacterium, the effect of NaCl on xylanase activity was examined. The xylanase was able to retain 67% of its original activity in the presence of 200 g/L NaCl, indicating that the enzyme also possesses halotolerant properties. Additionally, the xylanase operates optimally in the pH range of 5 to 8 and at temperatures between 45–55 °C. Its maximum activity was detected at pH 6 and 45 °C.
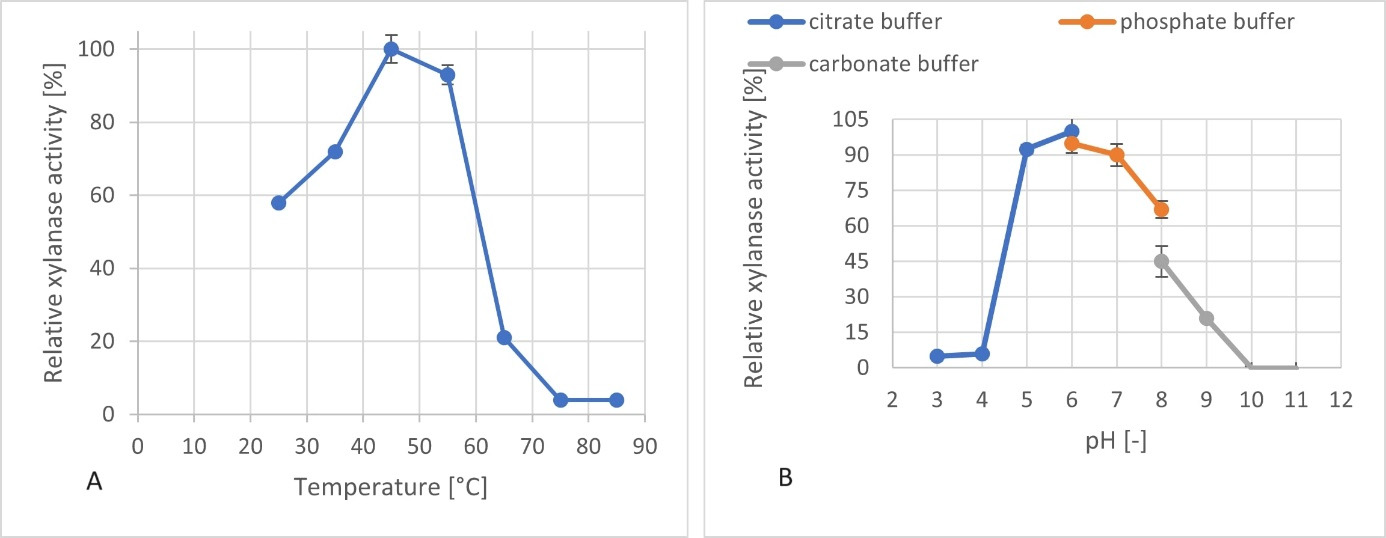
Figure 4. Relative xylanase activities at different temperatures (A) and pH values (B) measured in fermentation supernatant of C. phragmiteti cultivated in LB medium containing 1 w/w% of waste paper.
Based on the results of the enzyme stability tests, the xylanase is more stable at 45 °C compared to 55 °C. The enzyme retained nearly 60% of its original activity after 72 hours at 45 °C and pH 6. In contrast, the activities decreased to between 2–12% after 72 hours at 55 °C.
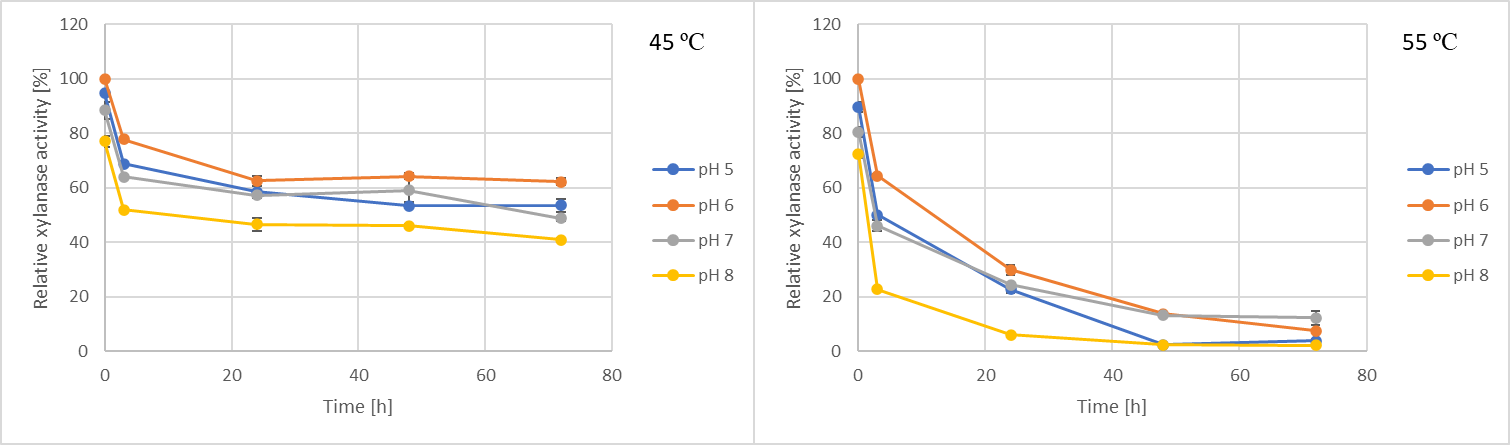
Figure 5. Results of the enzyme stability tests performed with xylanase produced by C. phragmiteti.
Investigation of hydrolytic enzyme activity and xylitol production by nonconventional yeasts
Xylanase activity was observed cultivating S. europaea and S. novakii on xylan, both on agar plates and in submerged cultures. However, further investigations are needed to determine the optimal conditions for xylanase activity measurement and enzyme production.
Xylitol production was observed with the following yeasts: S. novakii, K. molischiana, S. stipitis, C. allociferri, and C. baotianensis. The highest xylitol concentrations were performed by K. molischiana and C. baotianensis. Currently, we are focusing on optimizing xylitol production by C. baotianensis, and also planning to investigate the xylitol production of K. molischiana in a similar way in the future.
Table 1. Xylitol-producing strains and the measured highest xylitol concentrations
|
Nonconventional yeasts |
Highest xylitol concentration [g/L] |
Sampling time for the highest xylitol concentration |
|
K. molischiana |
16.7 |
48 hours |
|
S. novakii |
5.9 |
72 hours |
|
C. allociferri |
2.5 |
72 hours |
|
S. stipitis |
2.1 |
72 hours |
|
C. baotianensis |
18.1 |
72 hours |
Considering the limited available literature on the C. baotianensis yeast strain, our investigation is focused on determining the optimal conditions for xylitol production by this strain.
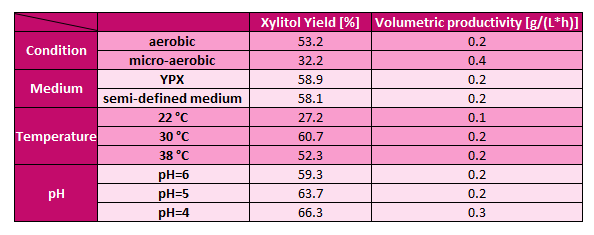
Figure 6. Results of the investigation of the optimal conditions for xylitol production by C. baotianensis.
The optimal conditions for xylitol production by C. baotianensis were identified as microaerobic cultivation at 30 °C and pH 4. During the comparison of different culture media, significant differences were not observed. Therefore, we chose the semi-defined xylose medium, as it contains xylose with minimal yeast extract and inorganic salts, making it a cheaper alternative than the YPX medium, which is rich in yeast extract and peptone.
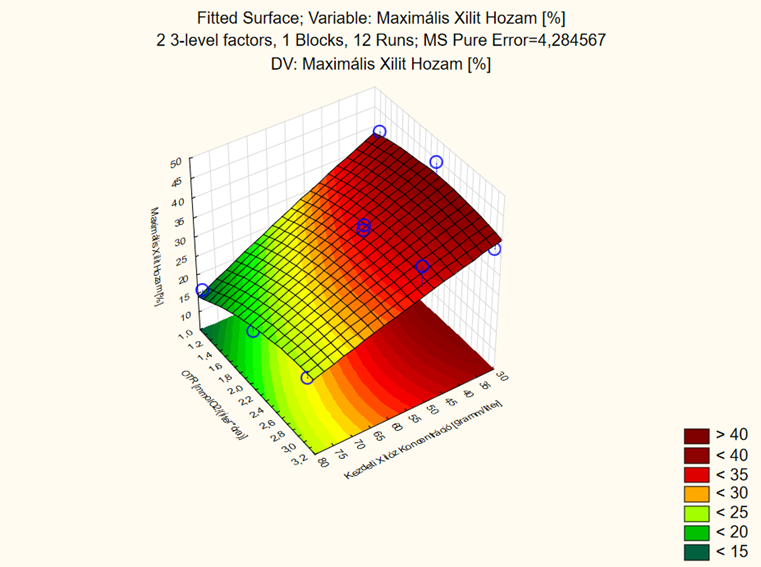
Figure 7. Surface fitted for maximal xylitol yield achieved by C. baotianensis.
According to the 32 experimental design, both the filling level of the flasks and the initial xylose concentration influence the maximal xylitol yield. To achieve higher yield, either lower (1–2.4 mmol O2/(L*h)) OTR values should be combined with lower (30–35 g/L) initial xylose concentrations or higher (2.4–3.2 mmol O2/(L*h)) OTR values should be combined with higher (50–55 g/L) initial xylose concentrations. The verification of the obtained model is in process.
Expected impact and further research
Nowadays, sustainability and environmental protection are increasingly important topics. The implementation of circular bioeconomy, operating and developing lignocellulosic-based biorefineries through integrated bioprocessing can provide a solution for the more cost-effective and environmentally friendly production of valuable products.
In the future, we aim to decompose agricultural and industrial by-products, and then convert the sugars obtained from the decomposition into valuable products (such as xylitol) through integrated bioprocessing in laboratory-scale fermenters, using either monoculture or mixed cultures. Potential candidates in mixed culture solutions could include C. phragmiteti, with its capability of xylanase production, and C. baotianensis, known for its excellent xylitol-producing ability.
Publications, references, links
List of corresponding own publications.
Kata Buda, Tünde Fekete, Ornella M. Ontanon, Eleonora Campos, Csaba Fehér (2024). Xylanase Production by Cellulomonas phragmiteti Using Lignocellulosic Waste Materials, Processes, 2024, 12, 258, https://doi.org/10.3390/pr12020258
List of references.
[1] Y.J. Bomble, C.Y. Lin, A. Amore, H. Wei, E.K. Holwerda, P.N. Ciesielski, B.S. Donohoe, S.R. Decker, L.R. Lynd, M.E. Himmel, Lignocellulose deconstruction in the biosphere, Curr Opin Chem Biol 41 (2017) 61–70. https://doi.org/10.1016/j.cbpa.2017.10.013.
[2] T. Hasunuma, F. Okazaki, N. Okai, K.Y. Hara, J. Ishii, A. Kondo, A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology, Bioresour Technol 135 (2013) 513–522. https://doi.org/10.1016/j.biortech.2012.10.047.
[3] S. Bedő, A. Fehér, P. Khunnonkwao, K. Jantama, C. Fehér, Optimized bioconversion of Xylose derived from pre-treated crop residues into Xylitol by using Candida Boidinii, Agronomy 11 (2021). https://doi.org/10.3390/agronomy11010079.