|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. KUPAI József
Application of bio-based organic compounds in catalysis
Introducing the research area
In many cases, organic chemists draw inspiration from natural compounds to develop new molecules that are suitable for a broad range of applications, starting from the pharmaceutical industry to catalysis. In my work, I have utilized bio-based molecules to create new chiral organocatalysts and a solvent suitable for various catalytic reactions.
Brief introduction of the research place
At the Organocatalysis Research Group, we believe that advancing new catalytic processes is crucial for the modern chemical industry. Catalysts not only conserve energy but also markedly enhance reaction selectivity. Our primary research interests encompass the application and recycling of organocatalysts in asymmetric synthesis and depolymerization reactions, along with the conversion and value-added utilization of diverse natural compounds.
History and context of the research
Natural compounds are playing a pivotal role in today's research. Plants harness carbon dioxide from the air through photosynthesis and produce a variety of complex, chiral molecules. These chiral molecules are part of the so-called chiral pool, which can only be synthesized via multi-step, stereoselective total synthesis, which could be a major challenge to chemists. Therefore, it is preferable to isolate these chiral compounds directly from biological sources, particularly from wastes (e.g., the bark of the trees), and use them as starting materials.
In my research, I have applied chiral organocatalysts. Organocatalysts are compact, metal-free organic molecules capable of facilitating organic chemical transformations. The significance of this field is underscored by the 2021 Nobel Prize in Chemistry awarded to David MacMillan and Benjamin List for their groundbreaking work in asymmetric organocatalysis. One of the advantages of asymmetric organocatalysts is their ability to influence the chirality of the resulting molecules. Chiral compounds are non-superimposable mirror images of each other, just like our two hands. This property holds immense biological importance; since depending on which mirror image pair is utilized, totally different effects can be observed. A compelling illustration of this is the sweetener aspartame, where one of the mirror image pairs is sweet while the other is tasteless. This is where the "magic" of organocatalysts lies, as they are capable of selectively producing only one of the mirror image pairs under optimal conditions.
The research goals, open questions
In my research, I aimed to develop new chiral organocatalysts. For the chiral units of these organocatalysts, I utilized a natural amino acid, (S)-proline, as well as cinchona alkaloids isolated from the bark of the cinchona tree native to South America. These new organocatalysts were evaluated in pharmaceutically relevant asymmetric syntheses, including the Michael addition and Diels–Alder reactions. Since the synthesis of organocatalysts can be both time-consuming and expensive, a lipophilic, recyclable cinchona-based organocatalyst was synthesized. The recyclability of this catalyst was examined in the synthesis of baclofen, a drug with muscle antispasmodic properties. In my doctoral thesis, I aim to broaden the application scope of organocatalysts and investigate potential limitations in their use.
Additionally, my research sought to expand the palette of alternative solvents. For this purpose, I selected sesamol, derived from sesame seeds, as a starting material and produced MeSesamol by methylating the hydroxyl group of sesamol. MeSesamol is a colorless liquid at room temperature. MeSesamol was employed as a solvent in the aforementioned asymmetric organocatalytic reactions, and thanks to its great solubilization power, its application was expanded in polymer chemistry.
Methods
The compounds were prepared using preparative organic chemistry techniques. The reactions were monitored by thin-layer chromatography (TLC) and high-performance liquid chromatography-mass spectrometry (HPLC-MS) measurements. The products were purified through preparative thin-layer chromatography, recrystallization, and column chromatography. The structure and purity of the synthesized compounds were identified using nuclear magnetic resonance spectroscopy (1H-, 13C-NMR), infrared spectroscopy (IR), optical rotation spectroscopy, and high-resolution mass spectrometry (HRMS). The enantiomeric purity of the products from asymmetric reactions was determined by chiral HPLC. The core component of the chiral HPLC technique is the chiral column containing a chiral stationary phase (e.g., cellulose). A solution of the chiral product is passed through this column under high pressure (up to 200–300 bar). As the two mirror-image pairs (enantiomers) of the chiral molecule interact differently with the column's stationary phase, they pass through the column at different rates. Consequently, the enantiomers are separated from each other, enabling accurate determination of the enantiomeric ratio. The thermal properties of MeSesamol were determined using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), while the open-cup flash point was determined using a Marcusson apparatus.
Results
Synthesis of chiral organocatalysts
In my research, I synthesized novel chiral organocatalysts starting from the amino acid, (S)-proline, and quinine. To enhance the efficiency of organocatalysts, a lipophilic catalyst was also prepared, which has neglectable solubility in polar solvents, allowing its easy recyclability by simple filtration (Figure 1).
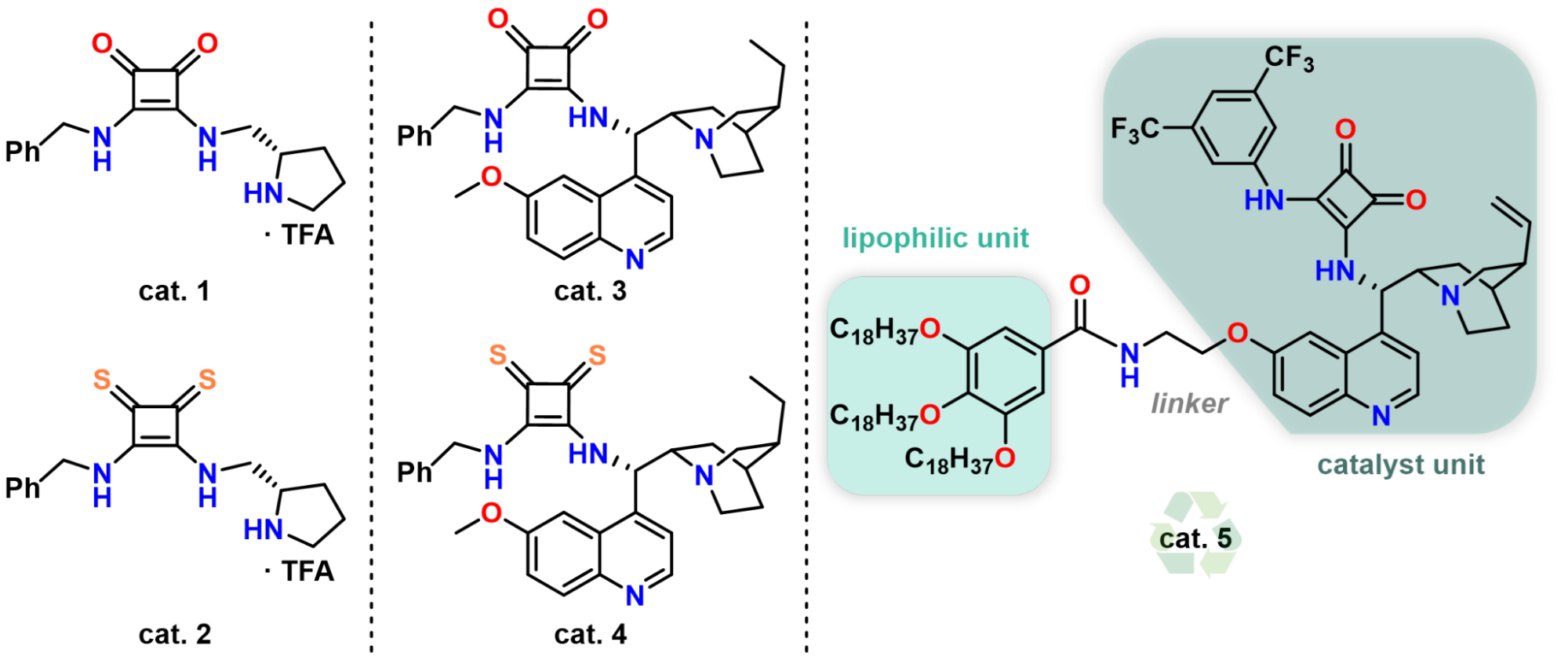
Figure 1. Chiral proline- and cinchona-based organocatalysts
Application of organocatalysts
Organocatalysts were employed in asymmetric syntheses. These reactions are highly significant in the pharmaceutical industry, since through asymmetric reaction, we can produce only one of the two mirror images of a chiral molecule. The selectivity of a catalyst is typically characterized by the enantiomeric excess value. The higher this value, the greater the selectivity of the catalyst. Proline-based organocatalysts have been studied in Diels–Alder reactions, which can be used to form complex ring systems with multiple asymmetric centers (Figure 2).
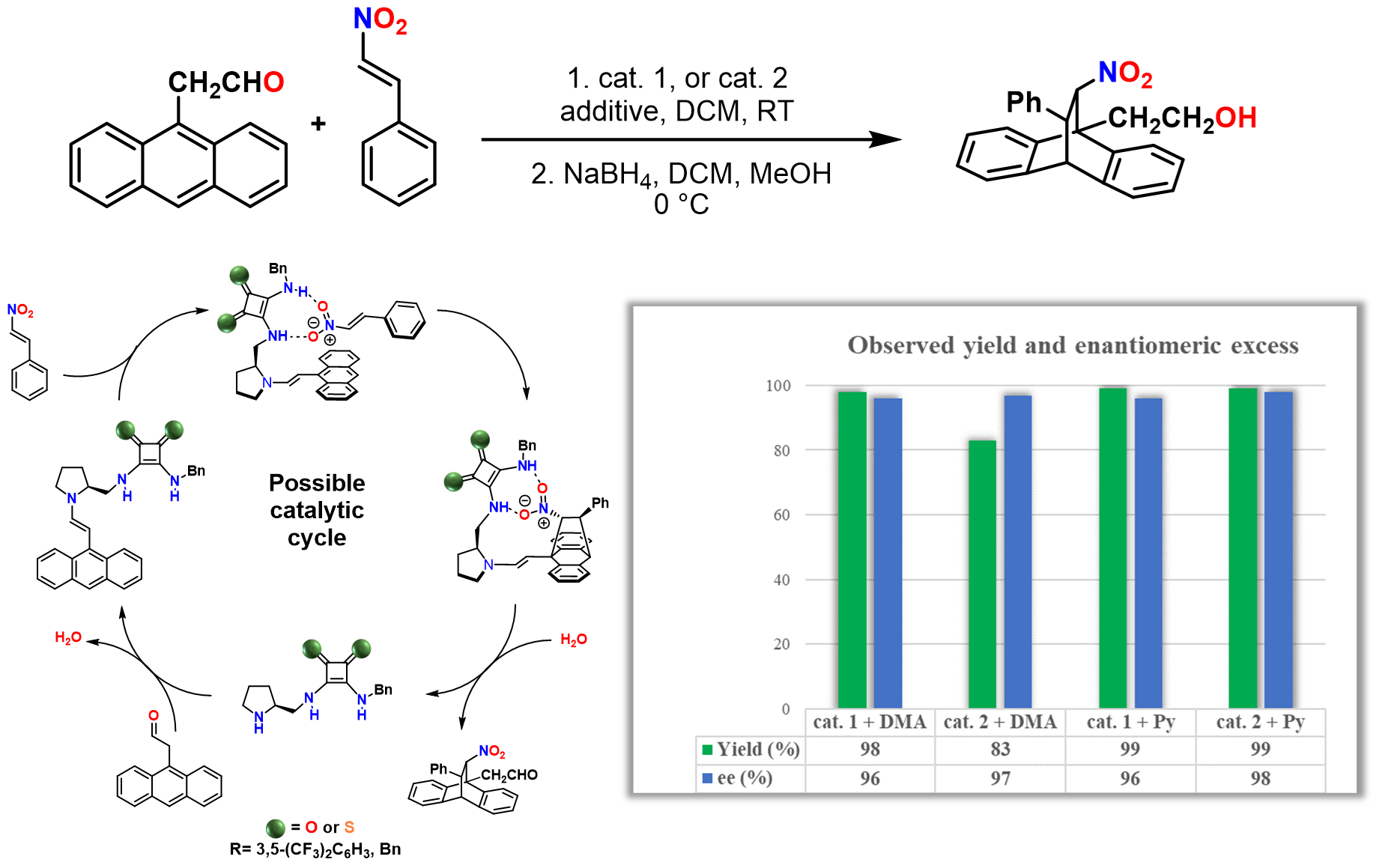
Figure 2. Application of proline-based organocatalysts in Diels–Alder reaction
In addition, the recycling of the lipophilic organocatalyst was explored in the synthesis of the drug baclofen. After the reaction, the apolar solvent (either anisole or toluene) was removed, and a polar solvent (acetonitrile) was added. Since the polar solvent does not dissolve the lipophilic organocatalyst but dissolves the resulting product, the precipitated catalyst can be separated through simple centrifugation. This allows the catalyst to be reused in a new catalytic cycle (Figure 3).
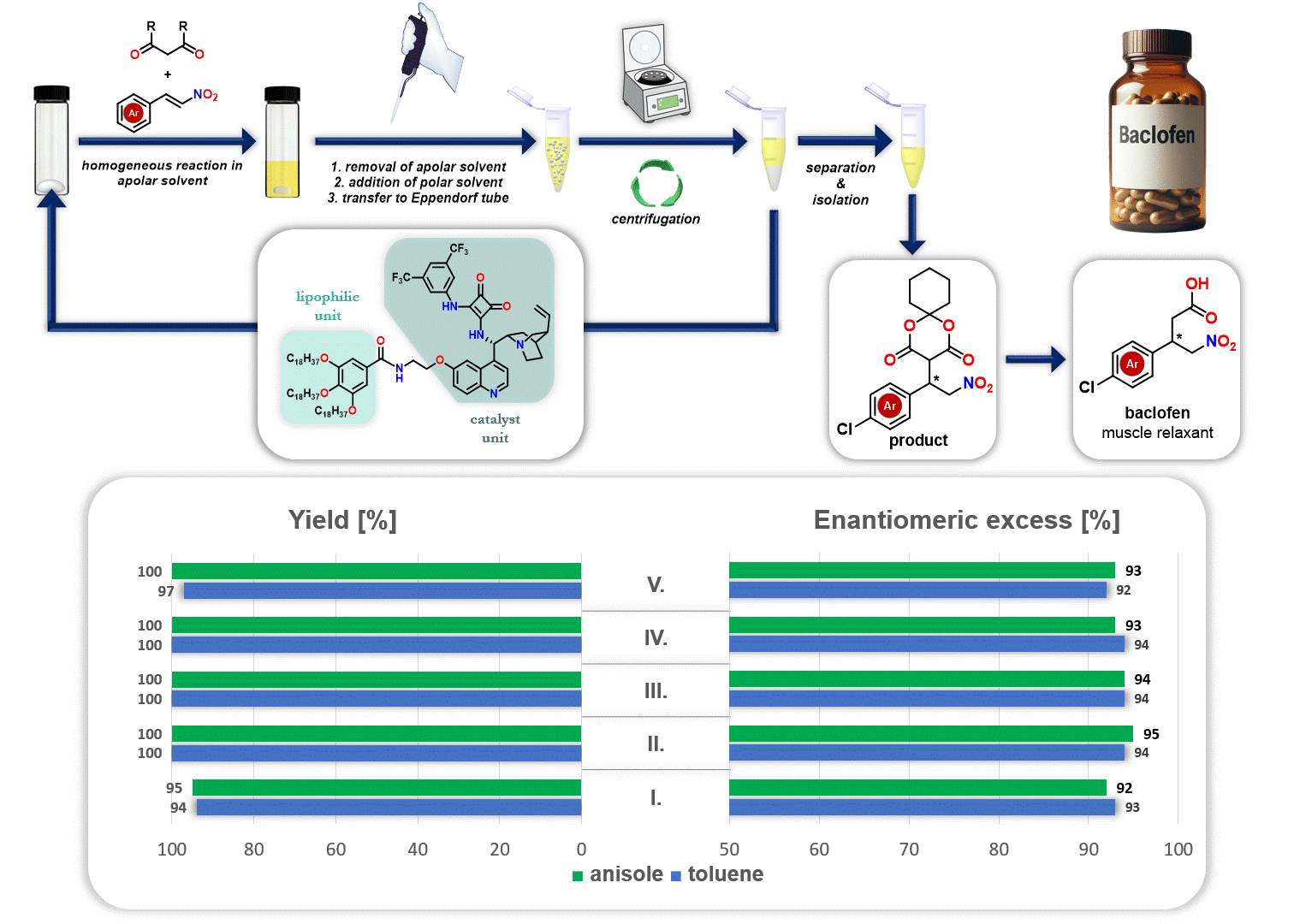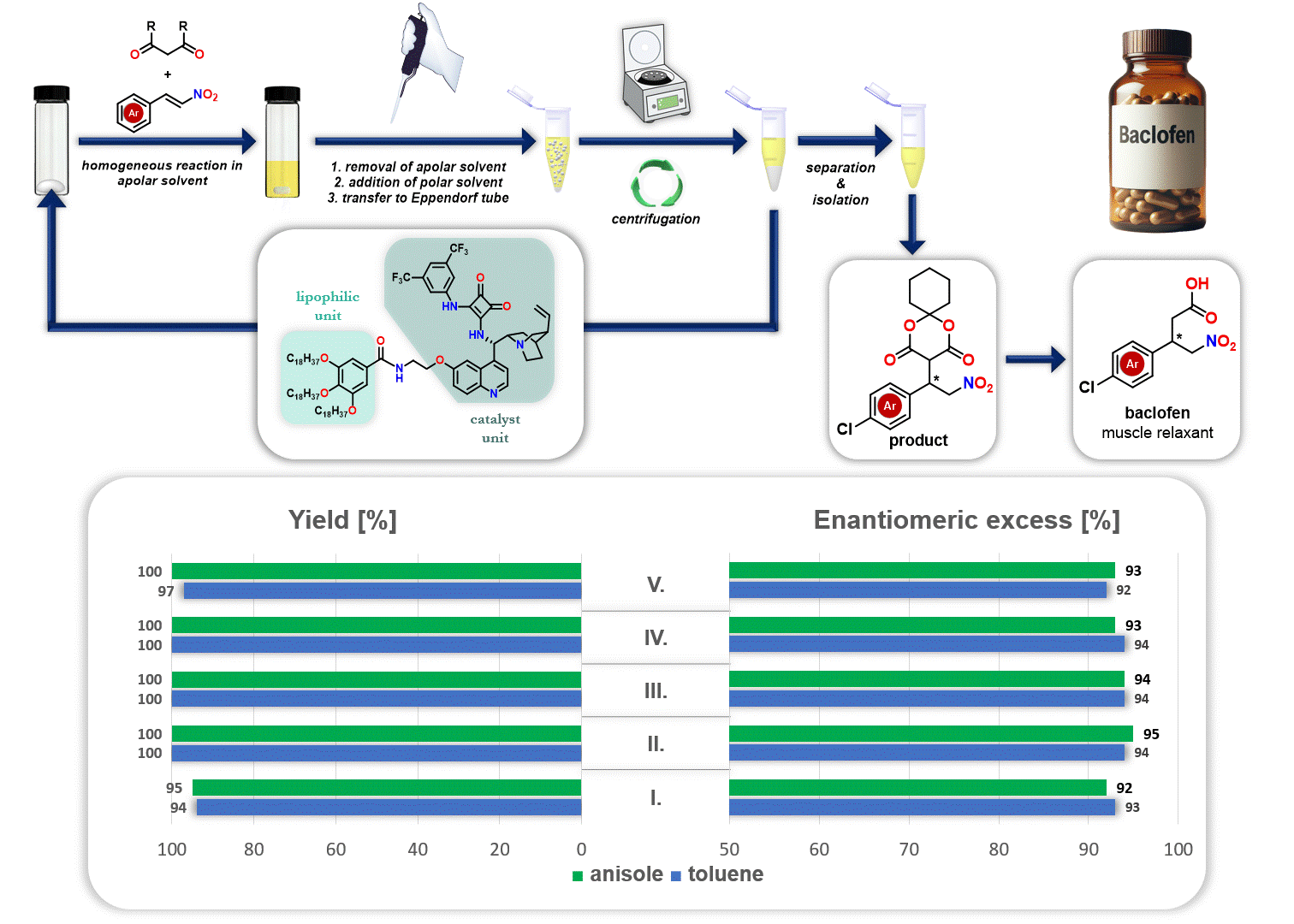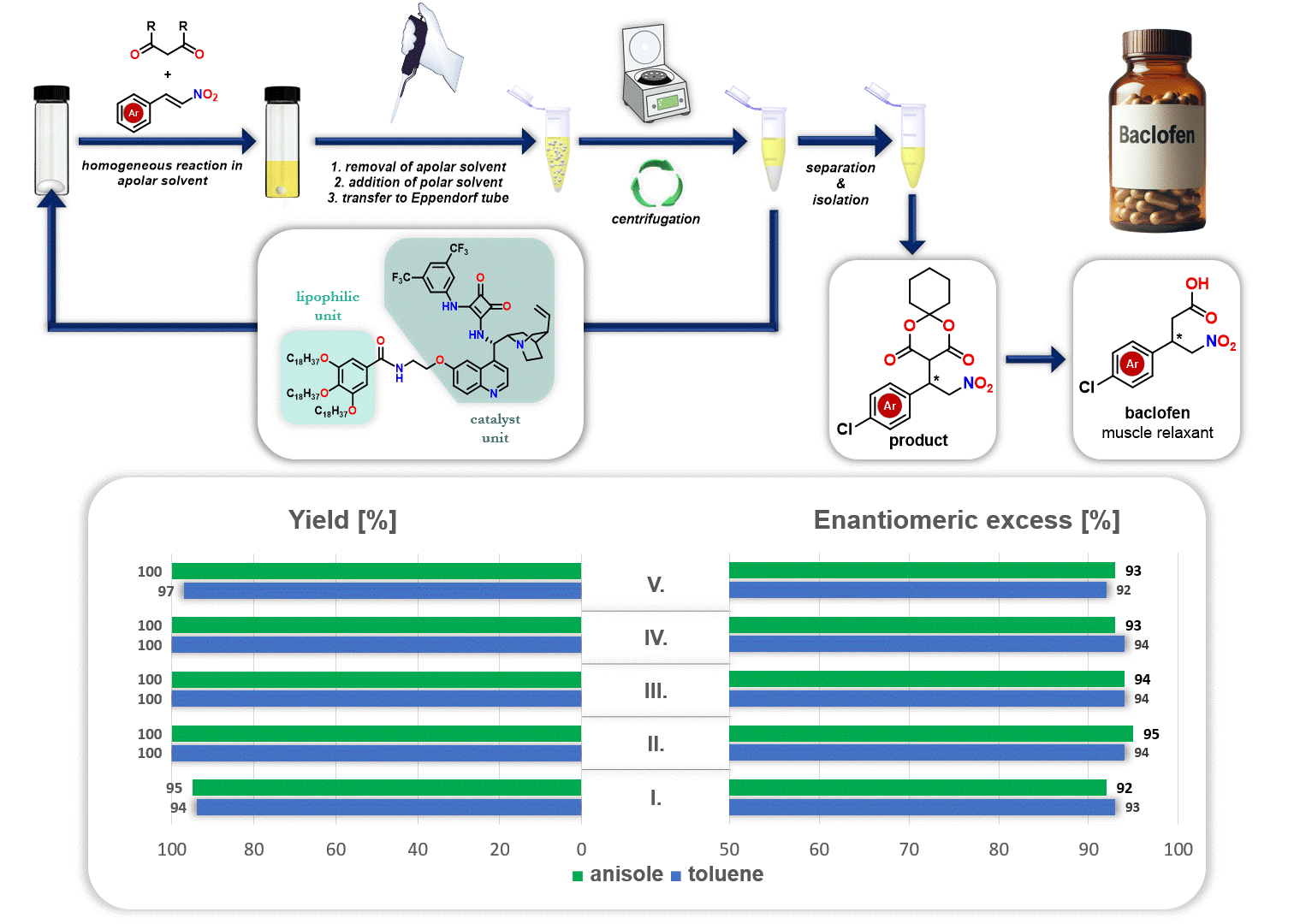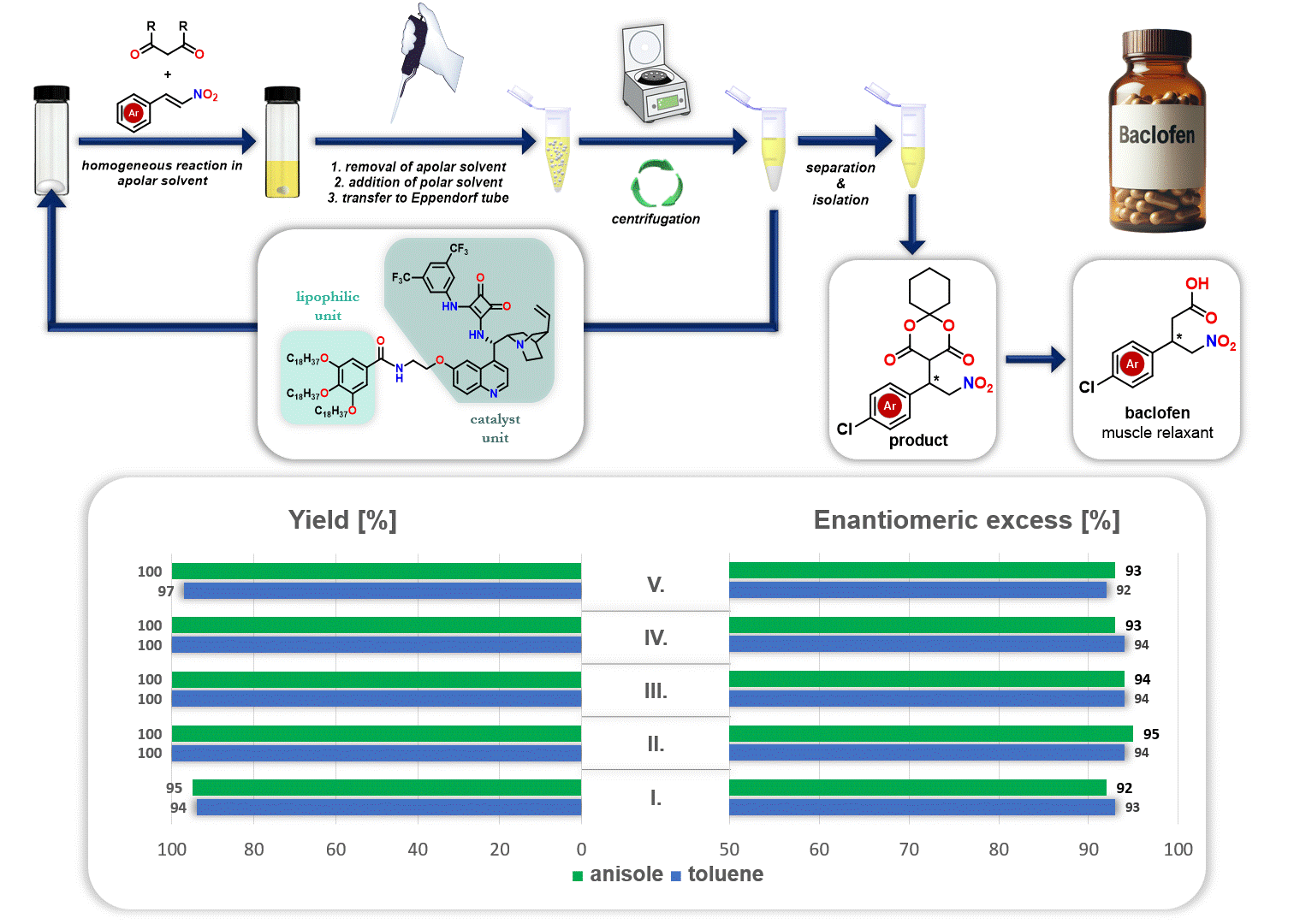
Figure 3. Recycling of lipophilic organocatalyst during the synthesis route of baclofen
These clearly show the role of proline derivatives and cinchona alkaloids in catalysis. Furthermore, cinchona alkaloids are known to possess biological activity. Their most renowned representative, quinine, has long been used as a drug for the treatment of malaria. Based on our studies, many of the cinchona alkaloid-based organocatalysts we have prepared also possess biological activity, therefore, their application in the future may hold great potential not only in catalysis but also in medicine.
MeSesamol: a new, bio-based solvent
Given the limited availability and environmental problems of fossil fuels, one of the greatest challenges facing humanity is to replace them with renewable raw materials. This is also the case for solvents, which represent a major part of the waste generated by the chemical industry. In my work, I have synthesized MeSesamol, a promising new bio-based alternative to polar aprotic solvents. MeSesamol has the advantage of being non-volatile, with a high boiling point of 229–230 °C and an open flash point of 123 °C; it is not miscible with water but is miscible with conventional organic solvents. MeSesamol has been successfully used as a solvent in various cross-coupling reactions, such as Suzuki and Sonogashira reactions, and asymmetric Michael additions.
Furthermore, MeSesamol has been demonstrated to be an excellent solvent for the depolymerization of poly(bisphenol A-carbonate) [BPA-PC] and poly(ethylene terephthalate) [PET]. The outstanding solubility properties of MeSesamol facilitated the depolymerization of these plastics into monomers at one of the lowest temperatures reported in the literature. Another advantage of using MeSesamol in depolymerization is its ease of recyclability, since after the reaction MeSesamol can be recovered by phase separation (Figure 4).
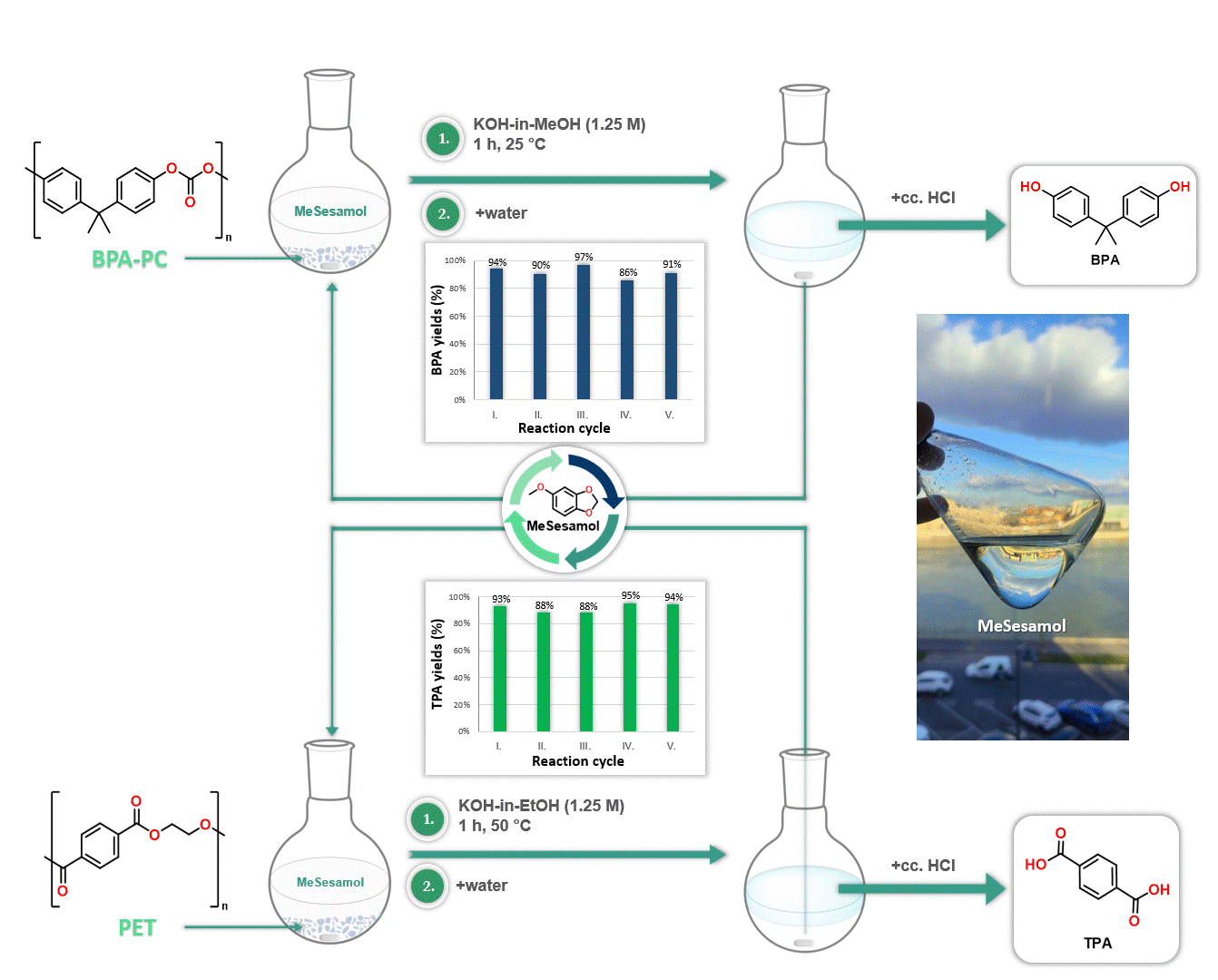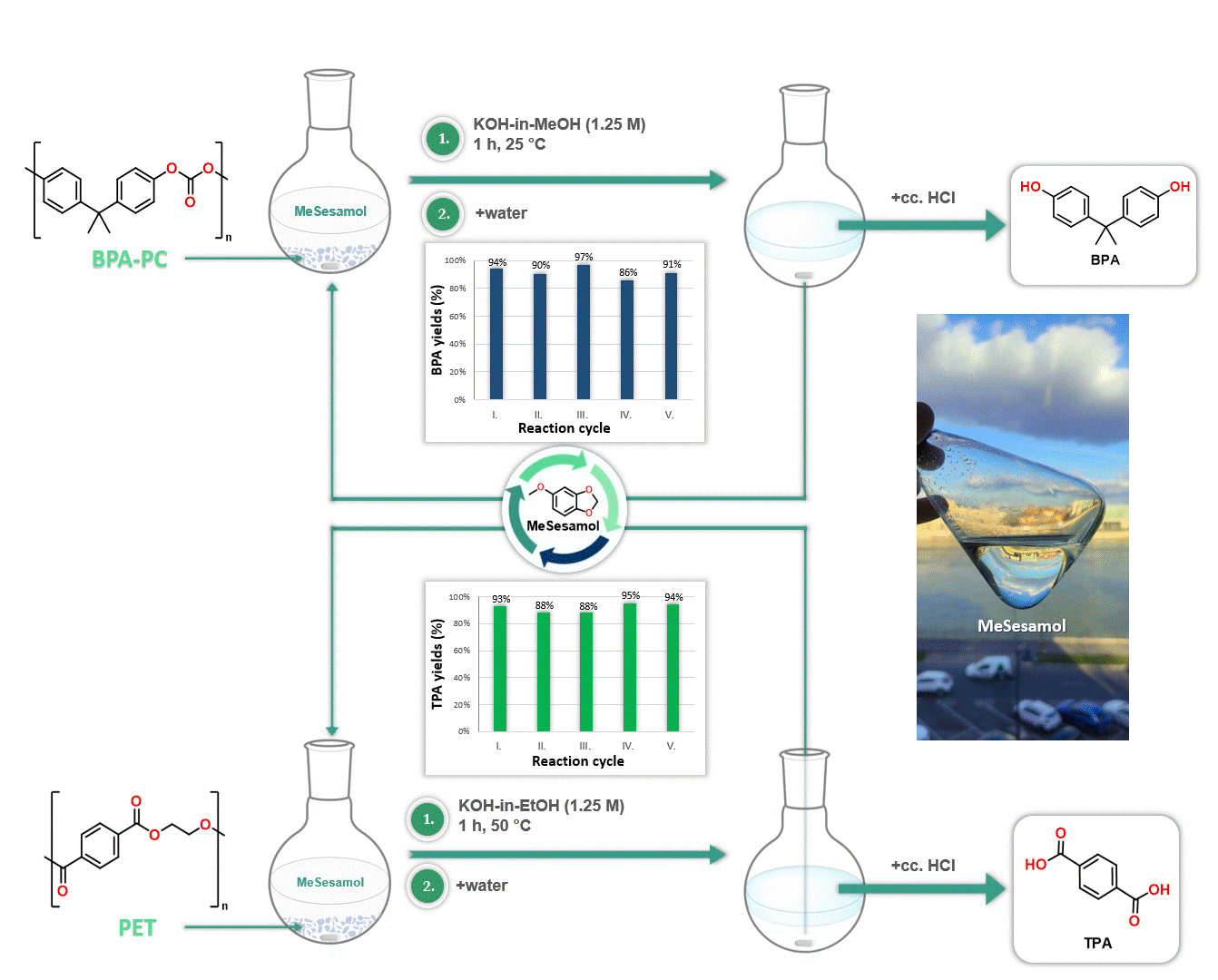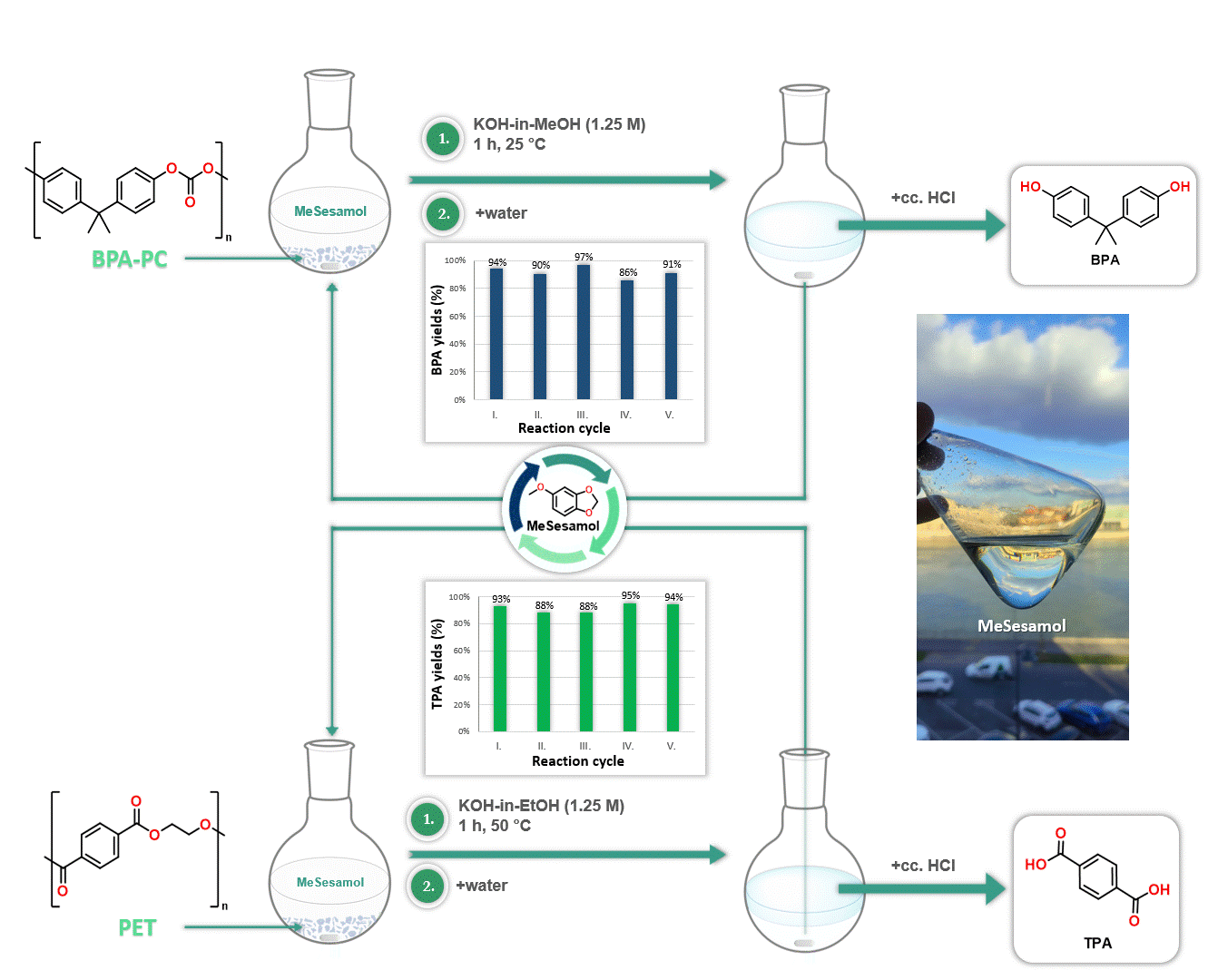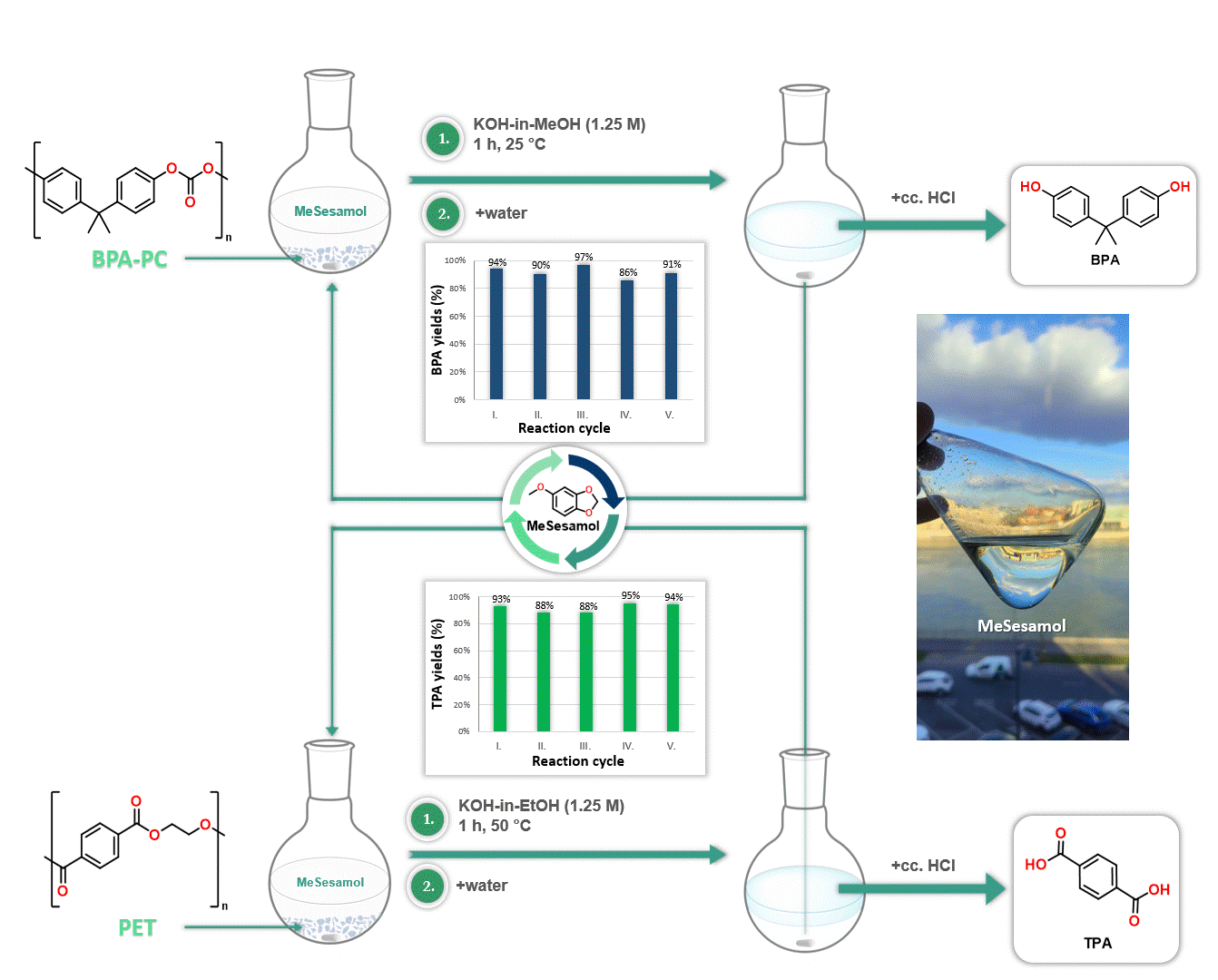
Figure 4. Application of MeSesamol as a solvent in depolymerization reactions
Expected impact and further research
The proliferation of asymmetric organocatalysts in the chemical industry has a significant impact. With their assistance, new types of drugs can be selectively produced, facilitating the development of novel molecules targeting new therapeutic targets. Their efficiency gains are not only felt in environmental terms but also economically, as they can reduce production costs. Research on organocatalysts is currently being pursued intensively, promising further advancements and deeper insights.
The development of new types of solvents could bring changes to the chemical
industry in many areas. Their application can reduce environmental impact and
enable safer and often more efficient reactions, paving the way for innovative
technologies. Our research group is currently exploring the development of new
organocatalysts and expanding the use of MeSesamol as a solvent.
Publications, references, links
List of corresponding own publications. (IF: impact factor, IC: independent citation)
Table of links.
List of references.
[1.] List, B.; Lerner, R. A.; Barbas, C. F. J. Am. Chem. Soc. 2000, 122, 2395–2396.
[2.] Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243–4244.
[3.] MacMillan, D. W. C. Nature 2008, 455, 304–308.
[4.] Castelvecchi, D.; Stoye, E. Nature 2021, 598, 247–248.
[5.] Marcelli, T.; Hiemstra, H. Synthesis 2010, 2010, 1229–1279.
[6.] Lu, T.; Wheeler, S. E. Chem. Eur. J. 2013, 19, 15141–15147.
[7.] Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Chem. Soc. Rev. 2021, 50, 1522–1586.
[8.] Burke, A. J. Expert Opin. Drug Discovery 2023, 18, 37–46.
[9.] Clarke, C. J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J. P. Chem. Rev. 2018, 118, 747–800.
[10.] Gu, Y.; Jérôme, F. Chem. Soc. Rev. 2013, 42, 9550–9570.
