
|
BMe Research Grant |

|
BME Kandó Kálmán Doctoral School
Faculty of Transportation Engineering, Dept. of Vehicle Manufacturing and Repairing
Supervisor: Dr. Antal Lovas
Absorption Storage of Hydrogen
Introducing the research area
Hydrogen storage is an important area of innovations primarily for the vehicle industry. Although clear vehicles – i.e. those exhausting solely stream – became technically feasible long ago, the spread of these vehicles was retarded by the high costs, low range and dangers involved in its application. In terms of safety and range, the main problem is the storage of hydrogen. Absorption storage (storing hydrogen in metal hydrides) may be an ideal solution for these problems.
Brief introduction of the research place
I perform my examinations in a laboratory of Dept. of Vehicle Manufacturing and Repairing (BME). A hydrogenating device was built for my work on the base of a Sieverts-type apparatus, which was upgraded to allow unique measurements. We collaborate with KFKI SZFKI (institute of Hungarian Academy of Sciences) and with the Solid State Department of Eötvös Lóránd University in sample preparation and evaluation examinations.
History and context of the research
The demand for energy by humans is covered mainly by fossil energy sources. At present the replacement of current energy industry is technologically not feasible. This is particularly true for transportation industry, which is nearly entirely based on "black gold". Though efforts have been made for turning transportation "greener", the great revolution (after which energy would be reached from renewable sources) is holding off.
Ensuring mobility is a great challenge for researchers as it is not only difficult to get the quite favorable properties of present fuels but also hard to make it economically.
An optimal solution is using hydrogen as a propellant:
on the one hand it can be utilized in a fuel cell via a totally renewing and environmentally sound method and we have nearly unlimited sources (water) – which could be converted into hydrogen using renewing energy sources (e.g. solar energy), on the other.
Application of hydrogen is dekayed by high costs and the absence of a safe storage method: Besides standard (high pressure) storage, 20 cylinders (50 liters, 200 bars) contain the amount of energy equal to a full fuel tank. This value can be enhanced by applying higher pressure (even 700 bars) or storing in liquid state and using hydrogen with high efficiency, but the range of present cars could only be reached with great compromises.
In the process of absorption storage hydrogen is absorbed by a metallic alloy. With this method the density of hydrogen (at room temperature) is higher than in liquid state. In exchange, storage material means extra weight – which could be reduced using light-weight metals.
Aim of the research
First, I try to understand the hydrogen absorption progress thoroughly. I study the impact of base materials, alloying elements, micro-structure, surface properties and several other factors on hydrogen uptake. There are two important attributes to be investigated: the amount of absorbed hydrogen and the rate of hydrogen-uptake. Absorbed amount of hydrogen is important because of the mass of the storage material: although hydrogen has high density in absorbed state, as the lightest element, it has usually insignificant mass ratio (lower than 1%) compared to the mass of storage material. The rate of absorption (optimal kinetics) is substantial for practical applications: our aim is to ensure the charging time as short as fueling up a present car.
After solving all of these problems, the task must be reversed: we have to empty the storage hydride, which means that we have to regain hydrogen, make absorption process reversible. Usually low pressure (vacuum → 1 bar) and elevated temperature is needed for this. Another important question is that how many times the charging-discharging cycle can be executed in succession: is there any structural degradation which leads to the decrease of storage capacity?
For a widespread application it is essential to produce the absorbing material economically in large quantities and without applying toxic materials.
Methods
A common method of storing hydrogen is high pressure storage, in gaseous state, but there are several other possibilities: in elementary state under ultra-high pressure or in liquid state, or chemically bound.
 The latter
includes different absorption methods (absorption in metallic alloys
(metal-hydrides), cryo- and room temperature absorption in carbon-nanostructures
etc.) as well as storing hydrogen in the form of compound. When choosing the optimal storing
method various factors have to be considered, like the consumption mode (amount, purity,
rate of consumption etc.), the attainable specific mass and volume, pressure,
temperature and many more.
The latter
includes different absorption methods (absorption in metallic alloys
(metal-hydrides), cryo- and room temperature absorption in carbon-nanostructures
etc.) as well as storing hydrogen in the form of compound. When choosing the optimal storing
method various factors have to be considered, like the consumption mode (amount, purity,
rate of consumption etc.), the attainable specific mass and volume, pressure,
temperature and many more.
The investigated metal-hydride storage excels with low specific volume and high capacity on low pressure, while specific mass (the ration of the mass of hydrogen and storage material) and the rate of hydrogen uptake and desorption should be improved.
Absorbing metals for use in absorption storage have to meet several requirements, which could only be performed through compromises and with alloying elements.
We can set out from theoretical capacity of base material, which means the amount of places in the atomic lattice that hydrogen can occupy. This is the theoretical maximum capacity of investigated material. It has two important index numbers: the mass ratio of hydrogen in the fully charged hydride (mass%), which is important for practical application and the so-called H/M ratio, which means the ratio of hydrogen and metal atoms in the charged hydride. The latter is important for chemical-structural considerations and because of easy comparison of materials. Certainly, maximum capacity depends on external physical conditions (pressure, temperature) as well. These conditions are usually determined so that they ensure the possibility of everyday use of the technology, for example 10 bar maximum pressure and 200°C maximum temperature is acceptable.
It is important, that the storage material desorbs as high proportion of absorbed hydrogen as possible. High value of reversibility is also important. Cycling means that how many times

the absorption-desorption cycle be executed repeatedly. It not uncommon that storage capacity decreases during cycling. Another relevant factors are the rate and characteristic of absorption and desorption – the kinetics.
My measurements were executed under hydrogen or argon pressure or under vacuum, between ambient temperature and 400°C in a self-made Sieverts-type apparatus. The principle of operation is based on the determination of absorbed hydrogen from the pressure-loss of gas space. Our apparatus is unique in that we can execute continuous (in-situ) resistance measurement during hydrogenation. As electric resistance shows a close correlation with hydrogen content and the structural changes of investigated material, I can execute measurements efficiently and accurately.
Investigated materials are Ni-Zr and Mg-based alloys in the form of a thin ribbon (see picture above). The former materials show quite good kinetics while Mg-based materials can reach high hydrogen-capacity.
Results
Determination ofthe maximum hydrogen content and the charging-discharging
kinetics of the investigated materials at different temperatures and pressures are the main targets of my examinations. They are complemented with further measurements to get information about the microstructure, local
composition, physical-chemical properties of the sample. I investigate the
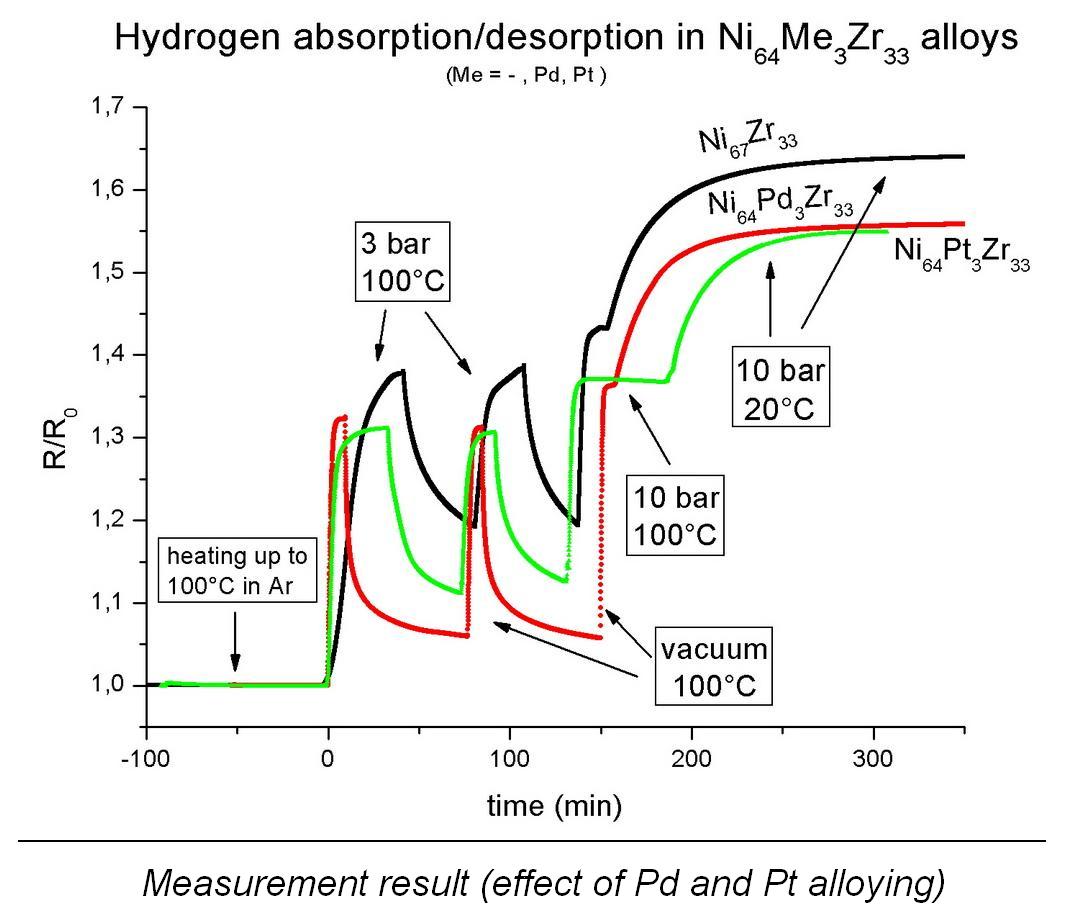
changes of these properties during hydrogenation as well. Thank to the construction of my apparatus, measured data can be recorded on a computer and I can analyze a resistance vs. time data series, where resistance (normalized to initial value) is proportional to the actual hydrogen content at every moment (see measurement results).
Large specific surface is important for absorption of hydrogen. The best way of ensuring would be using fine powders, however, the sample needs to be in bulky state for resistance-measurements. Therefore, thin (20–30 μm) ribbons were prepared by the so-called rapid solidification technology, where the molten alloy solidifies in a quite short period of time with a structure similar to frozen liquid (amorphous, glassy state) or with a nanocrystalline structure.
As a result, both specific surface and the diffusion rate of hydrogen are quite high in such structures. However, it is a metastable state, which is sensitive to high temperatures.
 I
reached quite fast charging-discharging kinetics at Ni-Zr-based alloys (3-5
minutes charging, 15-20 minutes discharging at 100°C; the same values are 30 and
120 minutes at 25°C). Due to their composition, these materials can absorb
maximum ~0,9 mass% hydrogen, of which 75% is absorbed reversibly, but this value depends on
the temperature – at higher temperature reversibility is higher.
I
reached quite fast charging-discharging kinetics at Ni-Zr-based alloys (3-5
minutes charging, 15-20 minutes discharging at 100°C; the same values are 30 and
120 minutes at 25°C). Due to their composition, these materials can absorb
maximum ~0,9 mass% hydrogen, of which 75% is absorbed reversibly, but this value depends on
the temperature – at higher temperature reversibility is higher.
I investigate the effect of cyclic charging-discharging on hydrogenation properties for these materials, for example I have executed a 50-cycle measurement which shows the stability of the structure (no decrease of charging capacity was observed). Diffusion and surface properties are also investigated for Ni-Zr-based materials.
Based on researches published literature and my earlier experiences, I started to examine Mg-based alloys. Magnesium is one of the most promising absorbing metal as one having a reversible hydrogen content exceeding 5 mass%, moreover it is present in large quantities in earth crust, and
can be processed relatively easily. Its main drawback is the high temperature (400°C) required for good kinetics. My aim is to decrease this temperature with appropriate components and microstructure. The first step is designing the alloys based on literature and my earlier results. These samples are prepared in a laboratory of Physical Research Institute (KFKI) with melt spinning technology. As a result, I have got a ribbon with a few hundredth of a millimeter in thickness. The microstructure, thermal stability and hydrogen uptake of these ribbons were investigated at different temperatures.
This part of the examination is quite new, I try to determine optimal heat-treatments and hydrogenation temperatures newly, though I have managed to indicate hydrogen uptake even at room temperature..
Expected impact and further research
Absorption hydrogen storage is a realizable technology; my aim to make this method practicable and economical. In accordance with this, my examinations target the development of a new, magnesium-based alloy and its optimal hydrogenation technology.
I would like to put my examinations into practice as well as realize a functioning hydrogen-operated system in our department, in cooperation with industrial partners. With this objective in mind, I try to extend my scope of activity to other elements of hydrogen-based (vehicle) systems (fuel cell, electromotors, regulation etc.).
Publications, references, links
Publications
B. Vehovszky, S. Balla: The Effect of Hydrogen Charging and Discharging in Zr(Ni,Pd,Pt,Cu) Glasses, Materials Engineering Vol. 15/2a, pp. 1–6, ISSN 1335–0803, Žilina, Slovakia, 2008
S. Balla, B. Vehovszky, A. Bárdos, M. Kovalaková: The study of
H-absorption-desorption process in Ni(67-X)MXZr33 glassy alloys monitored by in
situ resistance measurements, Journal of Physics: Conference Series vol.144,
012012, ISSN 1742–6596, 2009
B. Vehovszky, S. Balla: Resistometric and Volumetric Monitoring of Hydrogen-Absorption-Desorption Processes in Ni-Zr-Based Metallic Glasses, Journal of Machine Manufacturing Vol. XLIX. Issue E3–E5, pp. 35–38, HU ISSN 0016-8580, Budapest, 2009
J. Kovac, B. Vehovszky, L. Novak, A. Lovas: Viscous Phenomena in Magnetic and Thermal Properties of Fe-Ni-Based Glasses Induced by Cryo-treatments, IEEE Transaction on Magnetics Vol. 46 Issue 2. pp. 353–356, ISSN 0018-9464, 2010
B. Vehovszky, S. Balla: Effect of Surface and Bulk Properties on Hydrogen Absorption and Desorption in NiZr Metallic Glasses, International Journal of Applied Mechanics and Engineering, Vol. 15, ISSN 1425-1655, 2010
Conference presentations
The Effect of Hydrogen Charging and Discharging in Zr(Ni,Pd,Pt,Cu) Glasses – presentation, 25th International Colloquium on Advanced Manufacturing and Repairing Technologies in Vehicle Industry, May 26–28, Žilina, Slovakia, 2008
S. Balla, B. Vehovszky, A. Bárdos, M. Kovalaková: The study of H-absorption-desorption process in
Ni(67-X)MXZr33 glassy alloys monitored by in situ resistance measurements, The 13th International Conference on Rapidly Quenched and Metastable Materials, 24–29 August, Dresden, Germany, 2008
Resistometric and Volumetric Monitoring of Hydrogen-Absorption-Desorption Processes in Ni-Zr-Based Metallic Glasses – presentation, 26th International Colloquium on Advanced Manufacturing and Repairing Technologies in Vehicle Industry, May 28–30, Balatonfüred, Hungary, 2009
Hydrogen as an alternative propellant, problems of hydrogen-storage and a possible solution – presentation on Innováció és Fenntartható Felszíni Közlekedés (IFFK) (Innovation and Sustainable Surface Transportation, in Hungarian) conference, Sept. 3–5, 2009, Budapest, Hungary
Effect of Surface and Bulk Properties on Hydrogen Absorption and Desorption in NiZr Metallic Glasses – presentation, 27th International Colloquium on Advanced Manufacturing and Repairing Technologies in Vehicle Industry, May 19–21, Łagów, Poland, 2010
References
J.H. Harris, W.A. Curtin, M.A. Tenhover: Universal features of hydrogen absorption in amorphous transition-metal alloys, Phys. Rev. B, 36 pp. 5784–5797, 1987
Dr. János Prohászka: Bevezetés az anyagtudományba (Introduction to Material Sciences, in Hungarian), vol. I., Tankönyvkiadó, Budapest 1988
F. D. Manchester and D. Khatamian: Mechanism for activation of intermetallic hydrogen absorbers, Mat. Sci. For. Vol. 31, pp: 261–296, 1988
J. Garaguly: Hidrogén abszorpció-deszorpció vizsgálata amorf ötvözetekben, in-situ ellenállásméréssel (Investigation of Hydrogen Absorption/Desorption in Amorphous Alloys by Resistance Measurements, in Hungarian), PhD thesis, Budapest, 1998
K. Tanaka, Y. Kandaa, M. Furuhashia, K. Saitob, K. Kurodab and H. Saka: Improvement of hydrogen storage properties of melt-spun Mg-Ni-RE alloys by nanocrystallization, Journal of Alloys and Compounds Vol. 293–295, pp. 521–525, 20 December 1999
A. Züttel, P. Wenger, S. Rentsch, P. Sudan, Ph. Mauron, Ch. Emmenegger: LiBH4 a new hydrogen storage material, Journal of Power Sources 118 pp. 1–7, 2003
M. Y. Songa, S. N. Kwona, J-S. Baeb and S-H. Hong: Hydrogen-storage properties of Mg-23.5Ni-(0 and 5)Cu prepared by melt spinning and crystallization heat treatment, International Journal of Hydrogen Energy
Vol. 33, Issue 6, pp. 1711–1718, March 2008
K. Tanakaa, T. Miwab, K. Sasakib and K. Kurodab: TEM studies of nanostructure in melt-spun Mg-Ni-La alloy manifesting enhanced hydrogen desorbing kinetics, Journal of Alloys and Compounds, Vol. 478, Issues 1–2, pp. 308–316, 10 June 2009
Links
http://www.nature.com/nature/journal/v414/n6861/full/414353a0.html
http://www.hydrogencarsnow.com/mercedes-f600-hygenius.htm

