 |
BMe Research
Grant |

|
Pattantyús-Ábrahám Géza Doctoral School
Department of Polymer Engineering
Supervisors: Dr. Péter Nagy and Dr. László Oláh
Development of polymer-based implant materials for bone tissue engineering
Introducing the research area
During my PhD studies I investigated scaffolding materials for bone tissue engineering. The aim of such implants is to promote the regeneration instead of replacing the damaged tissue. After implantation, the scaffolds restore the main functions of host tissue, support the cell adhesion and proliferation. Parallel to the tissue remodeling, the implant material degrades and the degradation by-products metabolize out from the body.
Brief introduction of the research place
My work was carried out at the Department of
Polymer Engineering. Our department focuses on high quality work and research.
As a result, it was the first university unit in Hungary to introduce the
ISO 9001 Quality Management System in 2002. In 2004, the NAT (National
Accrediting Committee) also accredited our laboratory. The high-standard work is
reflected in the number of international publications, cooperations as well as
in the quality of our own journal. The eXPRESS
Polymer Letters is published by the department and is indexed by several
organizations, among others Thomson Reuters and Scopus. Its impact factor in
2010 is 1.575.
History and context of the research
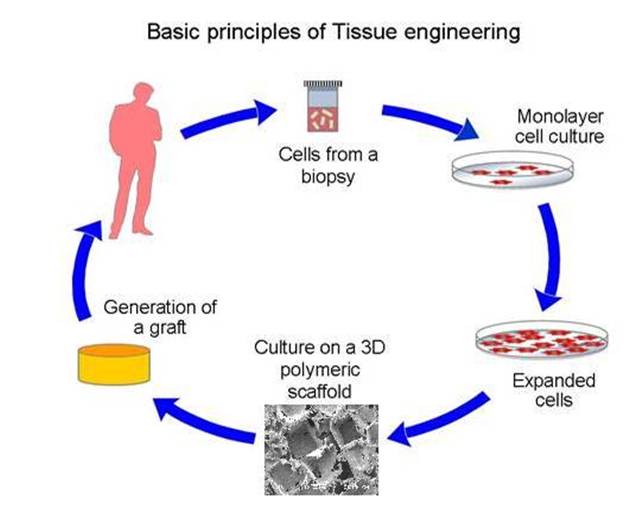 Tissue engineering (see scheme on the right), the restoration of the main
function of lost tissue is not a completely new idea. Studies in this topic were also
conducted in the early '80s. Moreover, skin reconstruction techniques developed at
that time are now in clinical use.
Tissue engineering (see scheme on the right), the restoration of the main
function of lost tissue is not a completely new idea. Studies in this topic were also
conducted in the early '80s. Moreover, skin reconstruction techniques developed at
that time are now in clinical use.
During the '90s a significant progress was made. The 2D cell cultures turned into 3D and the dimensions of scaffolds also increased [1]. Ever since, a number of materials, manufacturing methods and cell transplantation techniques were exposed. However, neither of them could meet all the requirements. The perfect method has not been invented yet.
The main functions of implant are to replace temporarily the role of lost tissue and to support the regeneration. For this purpose, adequate mechanical performance as well as bioactivity are needed. During the material selection the utmost important criterion is the biocompatibility, namely the compatibility with living tissues and organs. Since degradable polymers and ceramics are used, the degradation by-products should not elicit toxic materials or chronic inflammations.
The current in vivo and clinical experience have also pointed out that the main problems with biodegradable polymeric implants are the pH decrease in the surroundings of implant and the fragmentation of scaffolding material. The latter could generate post-operative problems after up to 5-7 years as well. Additionally, during bone remodeling, the small fragments could cause repeated inflammation and pain [2].
During the development of
scaffold manufacturing methods, techniques optimized for the processing of
individual or short series products were favored. This resulted in the
appearance of Rapid Prototyping technologies. The concept was to create implants
in one step based on a CAD model derived from CT (computer tomography) images.
The product could be either an implant or the underlying tool [3].
The research goal, open questions
The main aim of my research is to prepare tailored scaffold materials for bone tissue engineering. The implant should meet requirements similar to the properties of cancellous bone and support its load-bearing role for a period of 3-6 months. The gradual loss of implant properties necessitated the determination of degradation kinetics, as well. The deterioration of mechanical properties was, therefore, monitored on 3D scaffolds in vitro. Among the mechanical properties I paid special attention to the fracture mechanical behavior. The fragmentation of implant could be prevented by increased material toughness. As a result, the post-operative, late degradation inflammations could be avoided. In my study the manufacturing methods of individual, tailored implants and biocompatibility-related topics were also investigated.
Methodology
Applied materials
In my experiments biocompatible polymers (poly-ε-caprolactone, poly-D,L-lactide, lysine-isocyanates) and different salts (calcium-carbonate, β-tricalcium-phosphate) were used.

Preparation of scaffolds
After melt blending the scaffolds (see figure on the right) were prepared by hot-pressing. The porous structures were fabricated by melt-blending/particulate-leaching technique. Sodium-chloride was used as pore forming agent. After manufacturing, sodium-chloride could easily be leached out of the implant. Over 70% of salt content the scaffold has interconnected structure [4]. This form favors the ingrowth of cells into the implant and the application of toxic materials could also be avoided by using this manufacturing method.
Mechanical tests
The mechanical properties of examined materials
were characterized by quasi-static compression and tensile tests as well as by
fracture mechanical measurements. Since the applied polymers showed high
plasticity, the theories of linear-elastic fracture mechanics could not be
applied. For the characterization of fracture behavior the Essential Work of
Fracture (EWF) method was used. The basics of this concept are simple. It
separates the work performed within the plastic zone into a work required for
the formation of newly cracked surfaces – essential work of fracture term – and
into a work dissipated in the plastic zone – plastic or non-essential work of
fracture [5].
Fabrication tailored implants
The variation of medical cases calls for a cheap
and rapid manufacturing method of individual implants. The fabrication of such
implants was performed by a novel methodology. The concept is based on prior
methods, which used CT images to create the model of implant, and rapid
prototyping (RPT) techniques to fabricate the scaffold. In this study the mold
was prepared by RPT methods – this technique is called Rapid Tooling – and the
implant was formed by compression molding. During the development different
techniques were tested. 3D printers and Objet technique were also used. As a
result, the manufacturing process is fast – 1-2 days for dense implants and 1-2
weeks for porous scaffolds – and the prepared tools are much cheaper compared to
the conventional steel ones.
Degradation and biocompatibility studies
The degradation kinetics of applied polymers and
scaffolds were analyzed in vitro – under laboratory circumstances, at
37°C and in phosphate puffered saline. The effect of hydrolysis had been monitored
for two years. Structural, pH and mechanical property changes were studied. Cell
adhesion and proliferation studies were performed in cooperation with Semmelweis University 1st
Department of Pathology and Experimental Cancer Research Institute. The cell
ingrowth was analyzed in the Laboratory
of Experimental Gene Therapy of OVSZ (Hungarian National Blood Transfusion
Service).
Results
First, I would like to present my results relating
to the compatibilization of poly-ε-caprolactone/poly-D,L-lactide blends.
Subsequently, I deal with the composites filled with bioactive ceramics. Finally,
the results relating to the preparation of molds with Rapid Tooling are
presented along with the in vitro degradation and biocompatibility
tests.
Biocompatible polymer blends
 In the blends I
would like to combine the toughness of poly-ε-caprolactone with the higher
strength and modulus of poly-D,L-lactide. These two polymers are, however,
immiscible and their blends have inferior mechanical properties. The weak
interphase in the dispersed morphology cannot retard the crack propagation and,
as a result, the failure moves through these weak regions (see figure on the
left).
In the blends I
would like to combine the toughness of poly-ε-caprolactone with the higher
strength and modulus of poly-D,L-lactide. These two polymers are, however,
immiscible and their blends have inferior mechanical properties. The weak
interphase in the dispersed morphology cannot retard the crack propagation and,
as a result, the failure moves through these weak regions (see figure on the
left).
To improve the compatibility of the two phases,
reactive lysine-isocyanates were added to the system. Despite the small amounts
of compatibilizer (0.5 phr) the toughness increased significantly. The surface
stress, and as a result, the size of droplets decreased. The increase in fracture
resistance was prominent; in certain blends the essential work of fracture
nearly doubled.
Bioactive composites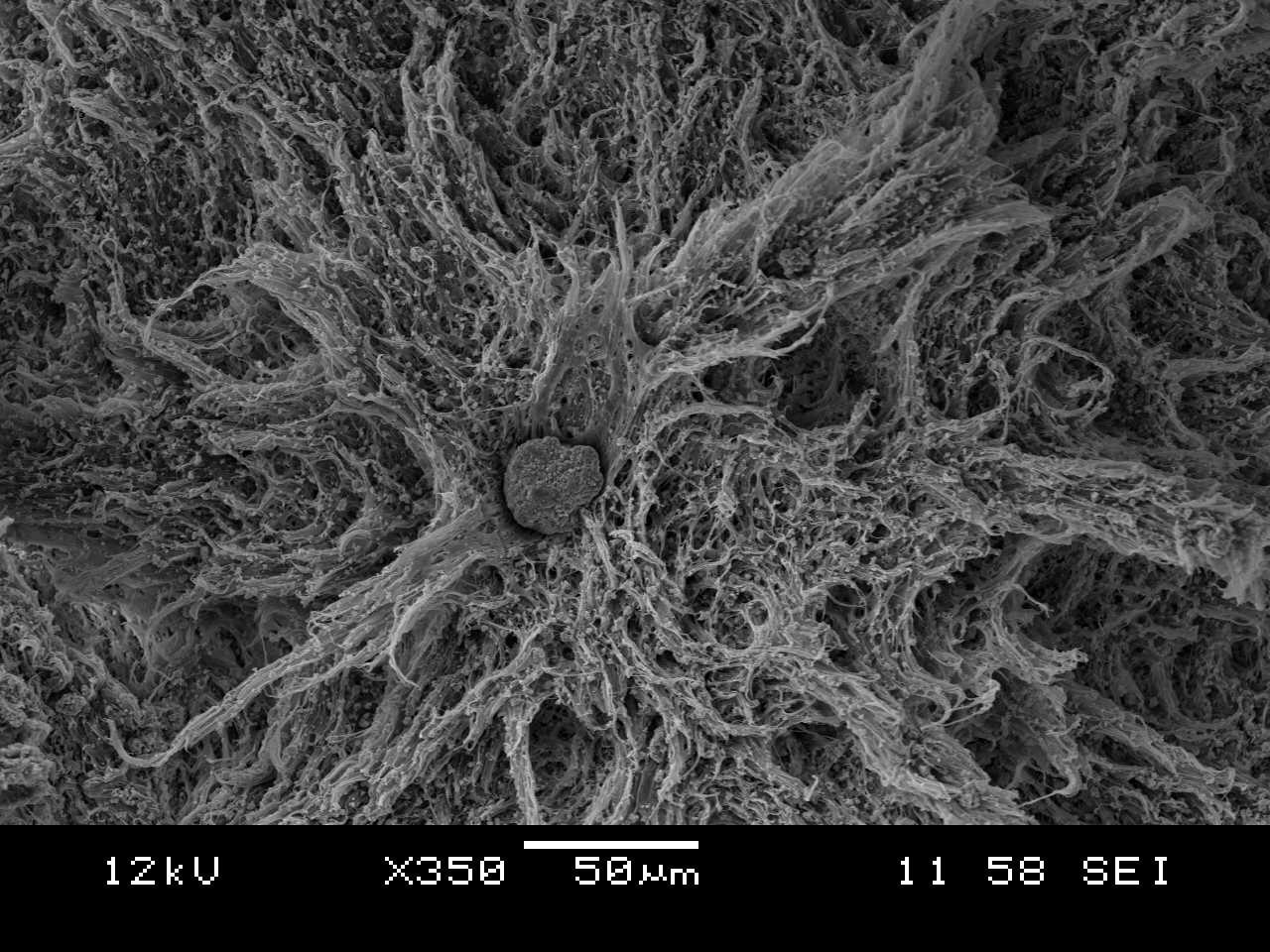
Composites containing poly-ε-caprolactone or
poly-ε-caprolactone/poly-D,L-lactide as matrix material and calcium-carbonate
and/or β-tricalcium-phosphate as reinforcement were also prepared. The goal in
these materials was to increase the mechanical strength and biocompatibility,
while maintaining adequate toughness. The effect of filler content and shape
(calcium-carbonate in the form of spherical calcite and needle-like aragonite)
was analyzed. Both calcium salts increased the compressive and tensile strength,
and the mechanical properties of cancellous bone were also attained.
Nevertheless, the filler content has an upper limit. The formed aggregates
(see figure on the right) demolish the material toughness.
Manufacturing of individual implants by Rapid
Tooling
The base materials were hot pressed in individual
tools to form implants with multiple curvature. The mold was manufactured by a
3D printer and an Objet apparatus (Figure 1) from the same 3D CAD model (the
base of this model was a former CT image), respectively. Both tools permitted
the preparation of poly-ε-caprolactone based scaffolds; however, the latter
technique facilitated opening the mold and the product removal. After compression
molding, to attain porous scaffolds, the salt was leached out from the implant
in two weeks.
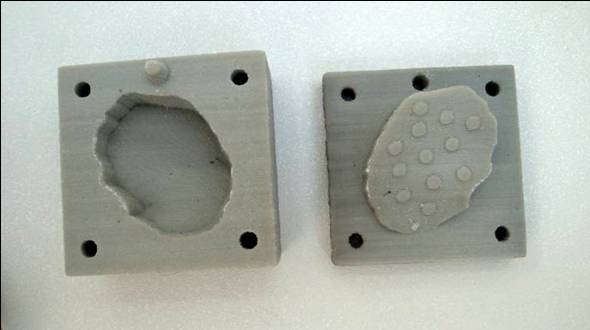
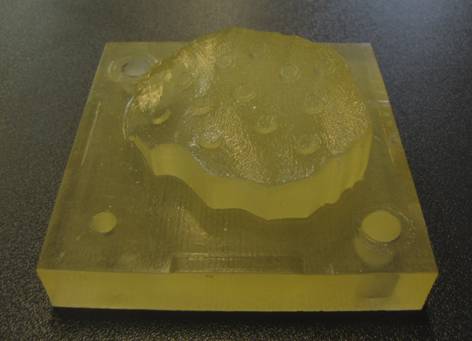

Figure 1: Molds fabricated by 3D printing and Objet technology as
well as the finished polymer implant
In vitro degradation studies
The degradation studies supported the hypothesis
that the hydrolysis of amorphous phase is faster than that of the crystalline parts.
This was demonstrated at certain time intervals with the increasing
crystallinity of samples. This process could be explained by the differences in
diffusion properties. The compressive strength and toughness of samples have also
deteriorated parallel to the decrease in average molecular weight. Differences
were observed between the degradation kinetics of porous and dense scaffolds.
This could also be correlated with the variation of diffusion
characteristics.
Biocompatibility tests
The bioactivity of calcium-carbonate salts was demonstrated with cell counting measurements. The cell adhesion on sample surface and the proliferation parameters increased with calcium-carbonate content. The reasons for this were the more hydrophilic surface of implant and its ability to stabilize the pH decrease resulting from the degradation of polymers.
The cell ingrowth was promoted in porous
scaffolds (Figure 2). This indicates that the prepared foams have interconnected
structure, and the pore size is large enough to allow cell ingrowth. The
fabrication of large cellular systems is, however, hindered due to the poor
vascularization and nutrition of inner regions.
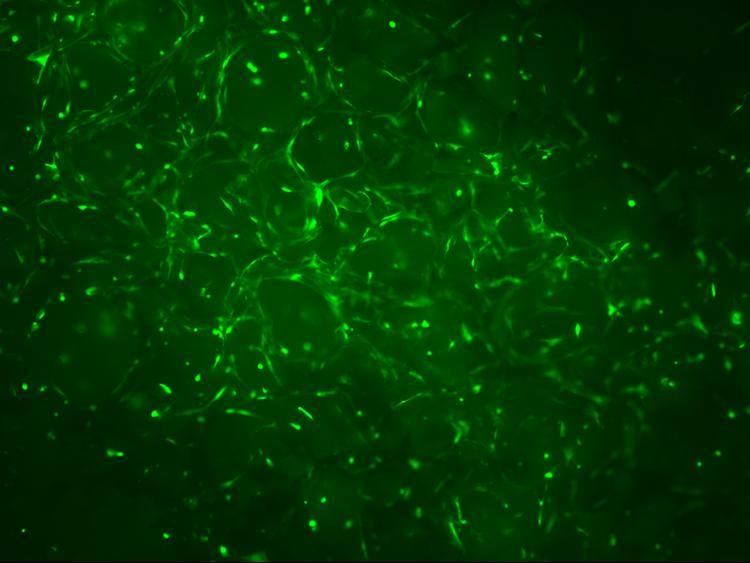
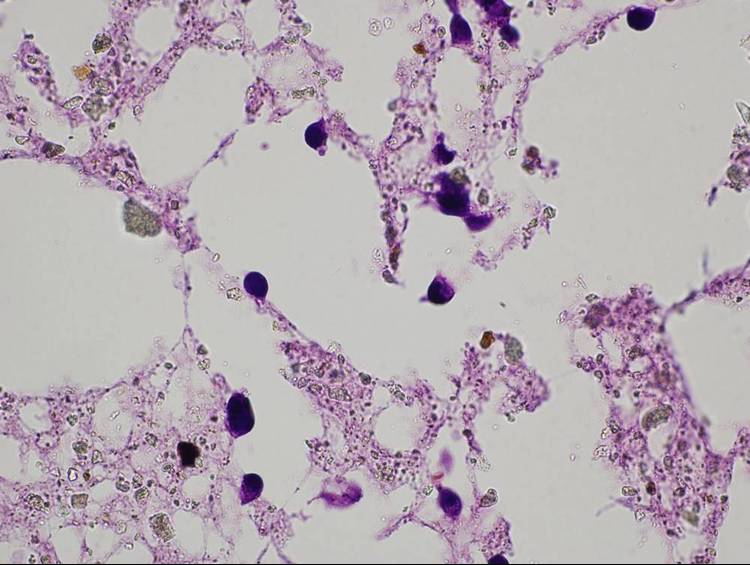
Figure 2: Cells in the pores of a scaffold (Fluorescent microscopic images with a magnification of 4x and 60x)
Expected impact and further research
I have prepared biocompatible polymer blends and composites, which have adequate properties to support major injuries in cancellous bone. These polymer blends of increased toughness can arrest the fragmentation of implants. Consequently, the embedding of small particles and late degradation inflammation can be avoided. Composites filled with bioactive ceramics can lower the post-operative inflammations caused by degradation by-products.
In the future I would like to combine the
toughness of blends with the bioactivity of composites. I plan to extend the
in vitro degradation and biocompatibility tests. In cooperation with
other research groups we may perform in vivo experiments, as
well.
Publications, references, links
Publications
Tuba F., Oláh L., Nagy P. Characterization of reactively compatibilized poly(D,L-lactide)/poly(ε-caprolactone) biodegradable blends by essential work of fracture method. Engineering Fracture Mechanic, (accepted with revisions)
Tuba F., Oláh L., Nagy P. Essential work of
fracture study of polymers – a novel criterion for the validation of tested
ligament range. Journal of Materials Science (accepted with revisions).
Tuba F., Oláh L., Nagy P. Characterization of fracture properties of aragonite and calcite filled poly(ε-caprolactone) by essential work of fracture method, Journal of Applied Polymer Science, 120(5), 2587-2595 (2011)
Oláh L., Tuba F. Investigation of calcium carbonates enhanced poly(ε-caprolactone) materials for biomedical applications. Macromolecular Symposia, 296(1), 371-377 (2010)
Szenti A., Tuba F., Kovács N. K. Rapid Tooling technologies in the processing of thermoplastic polymers. Materials Science Forum, 659, 97-102 (2010)
Tuba F., Borbás L., Nagy P., Oláh L. Hydrolysis induced deterioration of compressive properties of poly(e-caprolactone). FME Transactions, 37(1), 33-37 (2009)
Tuba F., Borbás L., Oláh L., Hadzima B. Development of polymer based scaffolds for guided tissue regeneration. Proceedings of the Third Hungarian Conference on Biomechanics (ISBN 978 963 06 4307 8), 379-386 (2008)
Tuba F., Oláh L. Development of polymer based biodegradable scaffolds for bone tissue regeneration – in Hungarian. Műanyag és Gumi, 44(4), 171-174 (2007)
References:
[1] Vacanti J.P., Langer R. Tissue engineering:
the design and fabrication of living replacement devices for surgical
reconstruction and transplantation. The Lancet, 354, S32-S34 (1999)
[2]
Bergsma J.E., de Bruijn W.C., Rozema F.R., Bos R.R.M., Boering G. Late
degradation tissue response to poly(L-lactide) bone plates and screws.
Biomaterials, 16, 25-31 (1995)
[3] Manó S., Novák L., Csernátony Z.
Application of 3D printing in cranioplasty – in Hungarian. Biomechanica
Hungarica, 1, 15-20 (2008)
[4] Oláh L., Filipczak K., Jaegermann Z., Czigány
T., Borbás L., Sosnowski S., Ulanski P., Rosiak J.M. Synthesis, structural and
mechanical properties of porous polymeric scaffolds for bone tissue regeneration
based on neat poly(ε-caprolactone) and its composites with calcium carbonate.
Polymers for Advanced Technologies, 17, 889-897 (2006)
[5] Bárány T., Czigány T., Karger-Kocsis J. Application of the essential work of fracture (EWF) concept for polymers, related blends and composites: A review. Progress in Polymer Science, 35, 1257-1287 (2010)
Links:

