 |
BMe Research Grant |

|
George A. Oláh Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Prof. László Poppe
Development, purification and novel applications of enzymes in chemical syntheses
Introducing the research area
Biocatalysis, in which organic compounds can be prepared by living organisms (mainly microorganisms) or enzymes, can be a modern alternative to the implementation of classic organic chemical reactions. Reduction of the length of multi-step synthesis routes could be realized by application of biological systems, usually under milder conditions (temperature, pressure, reaction time).
In my research work novel implementation opportunities of biocatalysis were investigated. The organic reactions carried out by biocatalysts were found more productive and selective (minimalization of the formation of the possible by-product).
Brief introduction of the research place
Bioorganic Research Group, at the Department of Organic Chemistry and Technology of BUTE, aims to investigate and extend the application possibilities of enzymes in organic chemical synthesis. In addition, basic research cooperation with academic (Institute of Enzymology, CRC, UBB) and industrial partners (ThalesNano, Fermentia, BIBUS) assure the feasibility of the projects’ scale-up with modern technologies in the view of the strictest environmental regulations.
History and context of the research
Nowadays economical synthesis of biologically active compounds is one of the major challenges of organic chemistry. Besides pharmaceutical industry, high enantiomeric excess in production is remarkably important for plastic, cosmetic and food industries as well. Enantiomers are molecules with same formula but with different configuration which are mirror images of each other but these are non-superposable. Most of the physical-chemical properties of two enantiomers are the same, but their biological activities and effects can be significantly different.
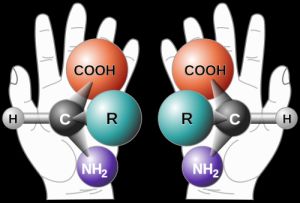
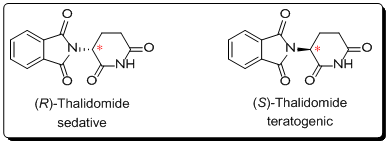
Presence of an undesired enantiomer could result in considerable problems. One of the most memorable example was the Contergan-scandal case where (R)-Thalidomide (which is a sedative) was not separated from its enantiomer (S)-Thalidomide which proved to be teratogenic. Contergan was commercially available from 1957 to 1961 when 12 000 children were still-born or born with truncated limbs.

Enzymes are proteins made of chiral amino acid, and therefore two enantiomers of the same molecule can be distinguished in a chemical reaction: one of them will be converted thereby the separation of useful and “polluter” enantiomers can be possible. Biocatalysis and biotransformation techniques have already been applied in the preparation of natural and non-natural amino acids, vitamins and further food-additives, as well as antibiotics, anticancer or antiviral drugs and other pharmaceuticals. In accordance with the requirements of our days, enzymes are environmental-friendly catalysts. The environmental regulations and quality standards require development of novel and increasingly efficient biocatalysts and biotransformations.
The research goal, open questions
Almost every type of synthetic reactions have an enzymatic counterpart, nevertheless cost of enzyme applications affected by the circumstantial preparation and purification of enzymes (downstream processes). In our research we aimed at the preparation and purification of enzymes with improved stability for novel applications in synthetic reactions considering industrial feasibility requirements.
Preparation of enzymes was carried out by fermentation of bacteria and filamentous fungus with the aim to determine the optimal parameters of enzyme production.
Usually the most expensive step of enzyme technologies is the extraction and purification of the enzymes from the crude fermentated media. Our aim is to develop solid enzyme supports which make downstream processes easier. Applications of purified enzymes are limited by the sensitivity to reaction conditions/circumstances and their shelf life is still low even under refrigerated conditions, which reduce or completely erode their activity. Aim of our research is to improve the enzymes’ stability, heat tolerance, mechanical properties as well as their activities and selectivities with novel immobilization techniques to be applicable for efficient synthesis of different, high purity organic compounds.
Due to their protein nature, enzymes only work in a narrow pH- and temperature range in aqueous media. Organic solvents or solvent-free media as well as extreme conditions (too low or too high temperature or pressure) are often required for enzymes in organic chemical synthesis (e.g. drug or pesticide production).Our aim is to extend the operating range of enzymatic systems by novel application methods (e.g. use of continuous-flow bioreactors).
Methodology
Synthesis of the compounds were carried out using modern methods of preparative organic chemistry. Purities were determined by thin layer chromatography (TLC), nuclear magnetic resonance (NMR) and infrared spectroscopy (IR).
Enzyme screenings were performed in batch mode in thermostated shakers. In thermostable equipments (numbers of simultaneous reactions can be tested up to 100) simplest implementation of numerous experiments might be assessed under the same conditions, at homogeneous temperature distribution and concentration in the reaction mixtures.
Experiments were performed in continuous-flow bioreactors such as ThalesNano X-CubeTM or in the in-house developed block-termostate based equipment capable of testing up to 20 bioreactors simultaneously under the same conditions. In these reactors, precise control of temperature, pressure and flow rate (-10-150°C, 0-150 bar, 0.1-3.0 mL/min) is feasible. The examined enzyme preparations were filled into pressure- and temperature-resistant stainless steel columns and then the solution of substrate(s) and reagent(s) were pumped through it from a reaction vessel. The effects of temperature, flow rate and pressure were determined. As a result, an easily generalizable procedure was developed which could be used in a wide range of substrates and reactions. It was found as an effective tool for the deeper interpretation of the mechanism of enzyme catalyzed reactions.
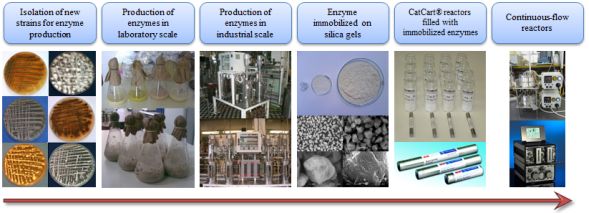
Enzymes adsorbed on different, surface-modified silica gel-based supports or entrapped into silica gel-based polymer matrices (sol-gel) were applied as the loading of the bioreactors. Surface modification of silica gels was performed in laboratory shakers at room temperature. Silica gel was suspended in alkaline water/methanol mixture then the surface was modified with different organosilanes (organic compounds containing carbon silicon bonds). The given solid supports have favorable mechanical properties and the examined enzymes were diversely bound on the different hydrophobic surfaces. Sol-gel cross-linked polymer matrices made from organosilanes can protect enzymes from environmental, physical, chemical and mechanical influences by encompassing them.
To follow up the reactions and for the determination of enantiomeric composition, chiral stationary phase gas chromatography (GC) or high-performance liquid chromatography (HPLC) methods were developed. These techniques apply a mixture flown through a stationary solid phase with a gas or fluid called mobile phase, and the various constituents of the mixture ‘travel’ at different speeds, resulting in separated compounds. If the stationary phase made of enantiomer-discriminative (chiral) material not only different chemical substances, but enantiomers of a molecule could be separated.
Results
Specific enzyme immobilization on solid supports can be a solution to avoiding the disadvantages of the above mentioned enzyme-properties(e.g.: purification, heat- and mechanical stabilization), however, swelling of the commonly used organic polymer-based supports (acrylic, styrene, divinylbenzene or polysaccharides) by liquids limits their applicability in continuous-flow reactors.
Silica gels as porous, high surface area, mechanically stable (up to 400 bar) supports can be suitable for purification and immobilization of enzymes in many ways. Its price is low enough for industrial use and as biocompatible supports are appropriate for biomedical applications. Activity and selectivity of the enzyme attached to silica gel could be influenced by the physical-chemical characteristics of the support. There is a hydrophobic interaction between the surface and the binding sites of the enzyme. Thus the enzymes become catalytically active.
Hydrophobicity, reactivity and specificity of silica gels could be fine-tuned by surface modification. Surface functionalization of numerous silica gels with different particle- and pore size were performed with more than 30 different reagents and reagent-mixtures. The supports gained in this way proved to be suitable to extract enzymes from crude fermentation of filamentous fungi Pseudozyma aphidis [A] and separation of enzyme mixtures (Candida antarctica lipase A and B) [B]. The slightly modified supports were able to make complexes with metal ions after further alterations. The altered supports can be thus applied for protein purification in affinity chromatography (IMAC, His6-tagged dUTPase enzyme) [K]. Contrary to the commonly used, commercially available affinity chromatographic supports our materials can be stored under milder conditions (no need of refrigerated storage under liquid) and they also worked properly with the less toxic lanthanides instead of toxic and carcinogenic nickel.
The physically attached enzymes on the surface of silica gel-based supports can be used directly in organic synthesis effectively. However, in aqueous media enzyme could be detached from the surface. With sol-gel enzyme immobilization it is possible to prepare more stable and heat resistant biocatalysts. It has been shown in the conversion of simple model-compounds that amendment of the components of polymer matrices provides an opportunity to improve the productivities and selectivities of the reactions with a biocatalyst which is application-optimized for the enzyme and also the reaction [C]. It has been demonstrated that compounds which are similar to the natural substrate of the enzyme improve the biocatalytic properties as the additive and the surrounding matrix strain the enzyme to immobilize with catalytically active conformation and preserve this rigid fixation [D].
Amines, alcohols and carboxylic acids are indispensable building blocks of drugs therefore, their production with high enantiomeric purity is a particularly important research area. We have successfully completed the production of these types of compounds in batch [E, F] and continuous-flow [G, J] modes. It has been verified in continuous-flow experiments that enantiomeric purity of the products is influenced by the structure of the substrate and the immobilization mode of the enzyme jointly [H].
Expected impact and further research
The results of our research group are related to international research collaborations (CMST COST Action CM0701) and constitute to the basis of domestic (KMR_12) and international (OTKA NN103242) tenders. Uninterrupted cooperation with our industrial partners ensures the possibility of the later large-scale implementation and that the research directions stay market-orientated all along.
Distant goal is to broaden of generalizability and application fields of the technologies based on our results [I]. Metal-bonding biocompatible supports, moreover the protein purification can be used remarkably for example in (heavy) metal decontamination from natural waters.
Within the framework of currently running projects, the entire automatization of designed protein purification and enzyme immobilization techniques with computer-controlled industrial robotic systems will be worked out.
Publications, references, links
Publications
Articles:
[A] M. Oláh, Z. Boros, P. Sátorhelyi, V. Bódai, E. Balázs, L. Poppe: Kinetic resolution of racemic 1-phenylethanamine catalyzed by lipase B from Candida antarctica – Effect of the acylating agent and the mode of enzyme immobilization, Stud. Univ. Babes-Bolyai, Chem, 2012, accepted for publication
[B] E. Abaháziová, Z. Boros, P. Kovács, L. Poppe: Surface modification of silica gels for selective adsorption of bacterial lipases, Stud. Univ. Babes-Bolyai, Chem, 2012, accepted for publication
[C] D. Weiser, Z. Boros, G. Hornyánszky, A. Tóth, L. Poppe: Disubstituted dialkoxysilane precursors in binary and ternary sol-gel systems for lipase immobilization, Process Biochemistry, 2012, 47, 428-434.
[D] G. Hellner, Z. Boros, A. Tomin, L. Poppe: Novel sol-gel lipases by designed bioimprinting for continuous-flow kinetic resolutions, Advanced Synthesis & Catalysis, 2011, 353, 2481-2491.
[E] P. Falus, Z. Boros, G. Hornyánszky, J. Nagy, L. Ürge, F. Darvas. L. Poppe: Synthesis and lipase catalysed kinetic resolution of racemic amines, Stud. Univ. Babes-Bolyai, Chem, 2010, 55, 289-296.
[F] J. Brem, M. Naghi, M. Tosa, Z. Boros, L. Poppe, F. Irimie, C. Paizs: Lipase mediated sequential resolution of aromatic β-hydroxy esters using fatty acid derivatives, Tetrahedron: Asymmetry, 2011, 22, 1672-1679.
[G] Z. Boros, M. Szigeti, A. Tomin, P. Kovács, L. Ürge, F. Darvas, L. Poppe: Asymmetric biotransformations in continuous flow reactors, Stud. Univ. Babes-Bolyai, Chem, 2009, 54, 69-75.
[H] Z. Boros, P. Falus, M. Márkus, D. Weiser, M. Oláh, G. Hornyánszky, J. Nagy, L. Poppe: How the mode of Candida antarctica lipase B immobilization effects the continuous-flow kinetic resolution of racemic amines at various temperatures, Journal of Molecular Catalysis B: Enzymatic, 2012, submitted
[I] P. Falus, Z. Boros, G. Hornyánszky, J. Nagy, L. Ürge, F. Darvas. L. Poppe: Reductive amination of ketones: novel one-step transfer hydrogenations in batch and continuous-flow mode, Tetrahedron Letters, 2011, 52, 1310-1312.
Patents:
[J] Poppe L., Tomin A., Boros Z., Varga E., Ürge L., Darvas F.: Új dinamikus kinetikus reszolválási eljárás (Novel method for dynamic kinetic resolution), Hungarian Patent Application, P0900720, 2009.
[K] Poppe L., Boros Z., Vértessy B., Kovács K., Tóth A., Hornyánszky G., Nagy J., Erdélyi B., Bódai V., Sátorhelyi P.: Fémkomplexáló ligandumokkal és stabilitásnövelő csoportokkal módosított felszínű affinitás anyagok és lantanida fémionok alkalmazása fehérjék megkötésére és tisztítására (Application of metal complexing and stability enhanced surface modified affinity materials and lathanide metal ions for immobilization and purification of proteins), Hungarian Patent Application, P1200327, 2012.
Lecture note:
[L] Poppe L. (ed.), Nagy J., Hornyánszky G., Boros Z.: Sztereoszelektív szintézisek, (Stereoselective synthesis; in Hungarian); Typotex Kft., 2011, ISBN 978-963-279-486-0
Links
BUTE Department of Organic Chemistry and Technology
