|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Dr György Marosi
Application of electrospinning and supercritical-fluid extrusion for the preparation of immediate-release pharmaceutical formulations
Introducing the research area
The innovative pharmaceutical manufacturing and analytical technologies investigated in this study harmonise with the novel approach that is supposed to characterise the industry in the 21st century. Taking several compounds as examples, we showed that extrusion assisted by supercritical carbon dioxide as well as electrospinning could save active pharmaceutical ingredients for healthcare where water solubility of these ingredients would otherwise hinder or disallow their application as medicine. Transmission Raman spectrometry, a modern analytical technique, proved to be capable of the characterisation of formulations.
Brief introduction of the research place
The Safety, Environmental and Pharmaceutical Technologies Research Group is committed to studies in material science and technology that are likely to improve quality of life. Researches targeting the development of safe, controlled, continuous and integrated pharmaceutical technologies, fire safety and application of renewable resources in automotive and aircraft industries and plastic waste recycling for environmental protection all serve this purpose.
History and context of the research
Increasing competition enforces pharmaceutical companies to revise their outdated manufacturing, development and quality approaches [1,2]. 36% of industrial expenses are spent for production [3], where rejection rate in certain processes can even reach 50%[4] which poses a huge burden on factories.
The solution for this problem can be continuous operation manufacturing lines the behaviour of which has been deeply understood using statistical methods and risk assessment before their actual application, which can be controlled and where the processed material is monitored in real time. The framework of such innovative development and production is outlined in the guideline of the American Food and Drug Administration (PAT) (Fig. 1) [5].
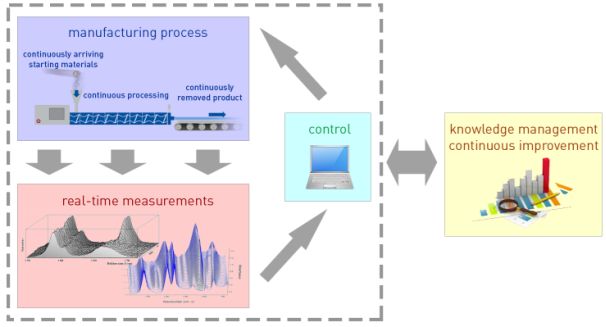
Fig. 1. Scheme of PAT-compatible development and manufacture
Electrospinning and supercritical fluid extrusion can become key elements of modern manufacturing lines when applied together with appropriate analytics. Besides, they can resolve important formulation issues, namely the poor water-solubility of new chemical entities.
The research goal, open questions
Goal 1: Dissolution improvement of drugs by preparing amorphous formulations with high specific surface area
90% of the recently discovered effective, typically crystalline, new chemical entities dissolve poorly in aqueous media [6]. For a systemic effect, the drug has to be dissolved in the body; furthermore, it must be absorbed into the cardiovascular system, which delivers it to its receptor. The bioavailability of problematic compounds can be ensured by special formulation (Fig. 2) [V5, V8].
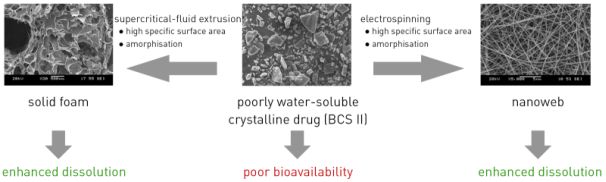
Fig. 2. Bioavailability enhancement of poorly soluble compounds with adequate permeability by supercritical fluid extrusion and electrostatic spinning
Goal 2: Hindering of precipitation in the body
When dissolution of a poorly water-soluble compound is enhanced, the orally administered drug may precipitate in the gastrointestinal system, due to too rapid dissolution causing local supersaturation. This can counteract absorption and make in vivo behaviour unpredictable. Our goal was to hinder precipitation by developing appropriate formulations.
Goal 3: Examination of the predictability of formulation properties from non-invasive analytical signal
According to the modern approach of pharmaceutical development and manufacturing, the processed material should be continuously monitored in real time. In our study, the drug-containing nanowebs and solid foams were examined using transmission Raman spectrometry, which can also be used in the preferred in-line mode.
Methods
Immediate-release formulations of poorly water-soluble crystalline drugs were prepared by means of electrospinning and supercritical-fluid extrusion, during which drug particles and molecules were dispersed in excipients. The properties of these excipients (matrices) enabled the formation of fibres and foams.
Preparation of nano- and microwebs
Active pharmaceutical ingredients (APIs) were embedded into nano- and microwebs using electrospinning (Fig. 3). The common solution of matrix-forming excipients and API was pumped through a spinneret under high voltage pointed to a grounded collector. The voltage induced formation of an ultra-thin jet from the spinning tip, resulting in the instant evaporation of the solvent and nanoweb with high specific surface area.
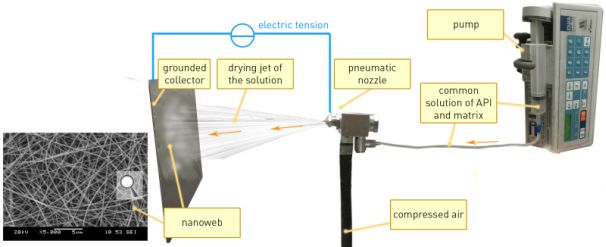
Fig. 3. Illustration of the electrospinning process
Preparation of solid foams
The mixture of API and matrix-forming substance was fed into a heated extruder (Fig. 4), where the polymer conveyed by a continuously rotating screw, melted and became plastic. This was mixed with supercritical carbon dioxide that turned into gas after leaving the instrument and foaming the plastic melt, which contained the API dispersed.
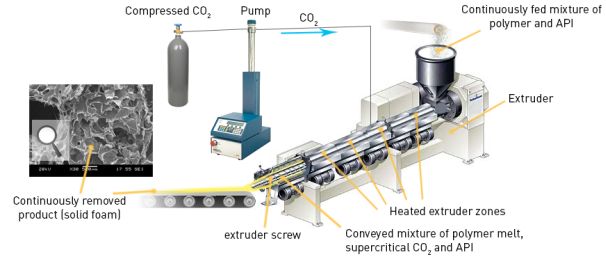
Fig. 4. Extrusion process assisted by supercritical carbon dioxide
Supercritical state of liquid carbon dioxide was reached by adjusting its pressure and temperature to above their critical values. Supercritical fluid properties resemble those of liquids as well as gases, because no separate liquid or vapour phases can exist under such circumstances.
Scanning electron microscopy
To characterize the product structure scanning electron microscopy was applied (Fig. 5). By scanning a selected part of the surface with a focused, narrow electron beam, the electrons escaped due to irradiation could be detected. By processing this signal, microscopic images could be obtained.
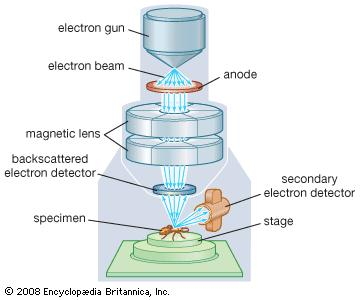
Fig. 5. Principle of scanning electron microscopy
© Encyclopædia Britannica, Inc.
Dissolution testing
Dissolution behaviour was tested using a standard tester instrument (Fig. 6). Dissolution vessels stirred with a paddle apparatus contained 900 milliliter of pH 1 hydrochloric acid in order to ensure gastric pH. At the beginning of the tests, webs or ground foams were placed in the vessel. At previously defined points in time the amount of dissolved API was determined using ultraviolet–visible spectrophotometry.

Fig. 6. Dissolution tester
Transmission Raman spectrometry
For transmission Raman spectroscopic measurements, the samples were irradiated with monochromatic light (785 nm) (Fig. 7), then the wavelength distribution of transmitted scattered photons was detected. Using mathematical methods, the chemical composition of the sample could be estimated, and the functional groups and their environment could be characterised. The spectrum obtained is characteristic of a sample volume that is much larger than what is illuminated. Thus, subsampling can likely be avoided.

Fig. 7. Principle of transmission Raman spectroscopy
Based on this graphics
Results
1. Dissolution enhancement of spironolactone by means of electrospinning
It can be desirable in many cases (migraine, high fever, heart problems and high blood pressure), that the orally administered drug compound be absorbed in a few minutes so that it can take instant effect. However, in the case of poorly water-soluble and well-permeable (BCS II) active pharmaceutical ingredients (APIs), immediate release can only be ensured by developing special formulations. Spironolactone, a BCS II steranic compound served a good model of these problematic APIs.
The electrospun amorphous nanowebs (Fig. 8) based on polyvinylpyrrolidon K90 (PVP) excipient had high specific surface area and contained no slowly dissolving crystal lattice (according to X-ray diffractograms and Raman spectra). These enabled the dramatic acceleration of API dissolution. However, it was followed by precipitation due to local supersaturations (Fig. 9). Addition of HPβCD complexing agent could hinder this phenomenon, at the same time enhancing dissolution further. High (40%)-drug-loading fibres could also be prepared, enabling full dissolution in two hours.
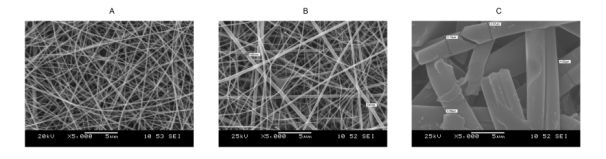
Fig. 8. Scanning electron microscopic photos of fibres with 20% spironolactone content
The matrices were: (A) PVP K90, (B) HPβCD/PVP K90 1:3, (C) HPβCD; Source: [V4]
Polymer-free spironolactone-containing HPβCD fibres could be prepared for the first time starting from ethanolic solution. The obtained fully amorphous web had excellent wettability, while the excipient could solubilise spironolactone. In this way, nearly total dissolution could be achieved in 1 minute, without precipitation. This implies that similar formulations may even be used as orally dissolving dosage forms. Drug molecules absorbed from the mouth can avoid first-pass metabolism in the liver.
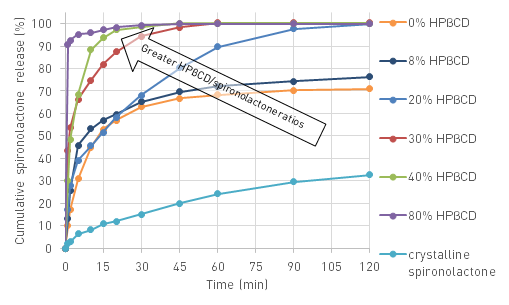
Fig. 9. Dissolution of electrospun fibres containing spironolactone in 20%, HPβCD in various amounts and PVP K90, compared to the slow dissolution of microcrystalline spironolactone; Source: [V4]
2. Dissolution enhancement of spironolactone by means of supercritical-fluid extrusion
Solid foams of spironolactone and prednisolone with few-micron-thick walls were produced, using Eudragit E polymer as matrix (Fig. 10) [V1, V3, V10]. Dissolution was reasonably enhanced in the case of these high-porosity formulations, too.
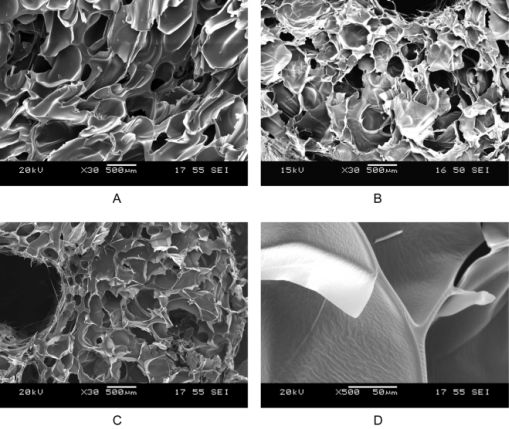
Fig. 10. Scanning electron microscopic photos of solid foams containing spironolactone in 10% and Eudragit E in 90%; Source: [V1]
Supercritical CO2 enabled decreasing the processing temperature and shortening of residence time, resulting in enhanced product purity, without significant decrease of the degree of amorphisation.
3. Application of Raman spectrometry to assess product properties
A key element of PAT-compatible innovative manufacturing is the detailed and real-time characterisation of products. In the example of spironolactone–Eudragit E extrudates it was proved with off-line measurements that Raman spectroscopy and chemometrics can enable estimation of product purity, degree of residual crystallinity and glass-transition temperature, which latter can influence physical stability (Fig. 11) [V2]. Using regression, the mentioned product properties were made predictable from the process parameter values. Spectra can be acquired in 1–2 minutes, which makes the technique suitable for real-time monitoring of production.
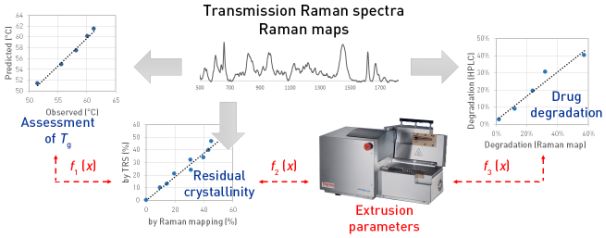
Fig. 11. Applicability of Raman spectrometry to estimate the purity, residual crystallinity and glass-transition temperature of extrudates; Source: [V2]
Expected impact and further research
The high potential in novel continuous technologies has yet been implemented in certain marketed pharmaceutical products [7,8] already manufactured by melt extrusion. The less studied supercritical fluid extrusion that needs special equipment has additional benefits. Therefore, our aim is to extend the scope of processed pharmaceutical ingredients and examine how supercritical carbon dioxide can aid their amorphisation.
Electrospinning can be employed in a wide range of manufacture, from formulation of bacteria [V7] through dissolution enhancement to preparation of supported catalysts [V6, V9]. Our aim with both technologies is the establishment of process control using analytical signals and hence realisation of PAT-compatible manufacture.
Lists of publications, references and recommended websites
Related own publications
[V1] T. Vigh, M. Sauceau, J. Fages, E. Rodier, I. Wagner, P.L. Sóti, G. Marosi, Z.K. Nagy: Effect of supercritical CO2 plasticization on the degradation and residual crystallinity of melt-extruded spironolactone - Non-invasive testing of immediate-release solid dispersions, Polymers for Advanced Technologies (2014) in press
[V2] T. Vigh, G. Drávavölgyi, P.L. Sóti, H. Pataki, T. Igricz, I. Wagner, B. Vajna, J. Madarász, G. Marosi, Z.K. Nagy: Predicting final product properties of melt extruded solid dispersions from process parameters using Raman spectrometry, J. Pharm. Biomed. Anal. 98 (2014) pp. 166-177
[V3] T. Vigh, Z.K. Nagy, M. Sauceau, E. Rodier, J. Fages, G. Marosi: Szuperkritikus szén-dioxiddal segített ömledékextrúzió alkalmazása gyógyszerkészítmények előállítására (in Hungarian), Műanyag- és gumiipari évkönyv (2014) pp. 67-72
[V4] T. Vigh, T. Horváthová, A. Balogh, P. L. Sóti, G. Drávavölgyi, Z. K. Nagy, G. Marosi: Polymer-free and polyvinylpirrolidone-based electrospun solid dosage forms for drug dissolution enhancement, Eur. J. Pharm. Sci. 49 (2013) pp. 595-602
[V5] A. Balogh, G. Drávavölgyi, K. Faragó, A. Farkas, T. Vigh, P.L. Sóti, I. Wagner, J. Madarász, H. Pataki, G. Marosi, Z.K. Nagy: Plasticized drug-loaded melt electrospun polymer mats: characterization, thermal degradation and release kinetics, J. Pharm. Sci. 103(4) (2014) pp. 1278-1287
[V6] P.L. Sóti, L. Telkes, Z. Rapi, A. Tóth, T. Vigh, Z.K. Nagy, P. Bakó, G. Marosi: Synthesis of an Aza Chiral Crown Ether Grafted to Nanofibrous Silica Support and Application in Asymmetric Michael Addition Journal of Inorganic and Organometallic Polymers and Materials (2014) DOI: 10.1007/s10904-014-0037-9
[V7] Z.K. Nagy, I. Wagner, Á. Suhajda, T. Tobak, A.H. Harasztos, T. Vigh, P.L. Sóti, H. Pataki, K. Molnár, G. Marosi: Nanofibrous solid dosage form of living bacteria prepared by electrospinning, eXPRESS Polymer Letters 8(5) (2014) pp. 352-361
[V8] H. Pataki, P. Soti, T. Vigh, Z.K. Nagy, B. Vajna, I. Csontos, G. Marosi: Controlled Formation of Free-Flowing Carvedilol Particles in the Presence of Polyvinylpyrrolidone, Chemical Engineering & Technology 37(2) (2014) pp. 249-256
[V9] P. Bakó, Z. Rapi, G. Keglevich, T. Szabó, P.L. Sóti, T. Vigh, A. Grün, T. Holczbauer: Asymmetric C-C bond formation via Darzens condensation and Michael addition using monosaccharide-based chiral crown ethers, Tetrahedron Letters 52 (2011) pp. 1473-1476
[V10] T. Vigh, M. Sauceau, E. Rodier, Z. K. Nagy, G. Marosi, J. Fages: Dispersion of an Active Pharmaceutical Ingredient in Eudragit E100 by Melt Extrusion Coupled with Supercritical Carbon Dioxide, 6th International Symposium on High Pressure Processes Technology, Belgrade, Serbia, 9th September, 2013
In: D. Skala, A. Dekanski (ed.) 6th International Symposium on High Pressure Processes Technology Proceedings, pp. 33-39, ISBN: 978-86-905111-2-9 (Belgrade, 2013)
Furthermore, 6 oral presentations and 3 posters in Hungarian and international conferences.
Recommended websites
- Guidance for Industry; PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance
- A review of hot melt extrusion
- Supercritical fluid
- Electrospinning
- Transmission Raman spectroscopy
References
[1] M. Birch, S.J. Fussell, P.D. Higginson, N. McDowall, I. Marziano: Towards a PAT-Based Strategy for Crystallization Development, Org. Process Res. Dev. 9 (2005) pp. 360-364
[2] S. Ferguson, G. Morris, H. Hao, M. Barrett, B. Glennon: Characterization of the anti-solvent batch, plug flow and MSMPR crystallization of benzoic acid, Chem. Eng. Sci. 104 (2013) pp. 44-54
[3] L. Abboud, S. Hensley: Wall Street Journal, New Prescription for Drug Makers: Update the Plants (2003) 2003. 09. 03, Dow Jones & Company, New York. Accessed: 29th June, 2014
[4] A.S. Rathore, H. Winkle: Quality by design for biopharmaceuticals., Nat. Biotechnol. 27 (2009) pp. 26-34
[5] U.S. Department of Health and Human Services, Food and Drug Administration, Guidance for industry: PAT – a framework for innovative pharmaceutical development, manufacturing, and quality assurance, (2004). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf Accessed: 29th June, 2014
[6] S. Bosselmann, R.O. Williams III: Route Specific Challenges in the Delivery of Poorly Water-Soluble Drugs, In: R. O. Williams III, A.B. Watts, D.A. Miller (eds.) Formulating Poorly Water Soluble Drugs p3, Springer, New York (2012) ISBN: 978-1-4614-1143-7
[7] https://www.ices.a-star.edu.sg/media/30880/5-Melt-Extruded-Oral-Controlled-Release-Systems.pdf Accessed: 29th June, 2014
[8] J. Breitenbach, Melt Extrusion Can Bring New Benefits to HIV Therapy: The Example of Kaletra Tablets, Am. J. Drug Deliv. 4 (2006) pp.61-64
