|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Organic Chemistry and Technology
Supervisor: Tünde TÓTH (PhD, associate professor)
Co-supervisor: Péter HUSZTHY (DSc, professor)
Preparation and Evaluation of Crown Ether Based Sensors Containing an Acridone Moiety and Selectors Containing an Acridine Subunit
Introducing the research area
Wastewater of household and industrial origin may contain significant amounts of toxic substances, including heavy metals, therefore the development of sensor molecules capable of selective recognition and selector molecules capable of selective binding of these substances is of great significance. During my research I have synthesized acridono-18-crown-6 ether based sensor molecules and I have also studied their complexation ability by UV-vis spectroscopy and single-crystal X-ray diffraction.
Brief introduction of the research place
In the Supramolecular Chemistry Research Group led by Professor Peter Huszthy, at the Department of Organic Chemistry and Technology, the research of crown ether based cation sensors and selectors for primary amines is being carried out. The complexation ability of these substances is evaluated at Gedeon Richter Plc., the Institute of Enzymology of the Hungarian Academy of Sciences, and the Department of Applied Biotechnology and Food Science.
History and context of the research
Supramolecular chemistry deals with the formation, the properties and the applications of molecular associations held together by non-covalent forces [1]. The origin of these molecular associations is based on the phenomenon of molecular recognition: in which case a molecule called a host selects an other molecule called a guest from the surrounding molecules and they form an organized structure (a complex) held together by secondary interactions (for example hydrogen bonding). Good examples for this generally occurring phenomenon in Nature are the selective metal ion binding and transport by natural ionophores (e.g. K+ selective Valinomycin) through biomembranes (Figure 1 A, B).
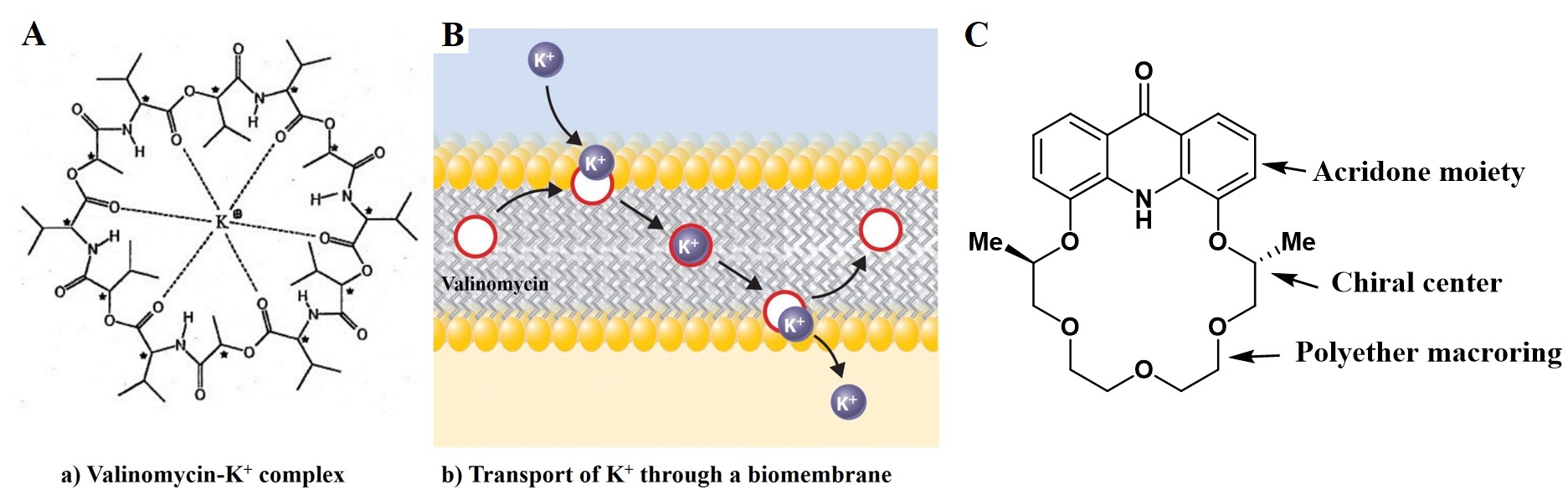
Figure 1
Nobel laureate chemist Charles Pedersen showed that molecular recognition is not the unique property of living organisms, but it can also be brought about by synthetic macro-cycles, such as the crown ethers, which were first synthesized and studied by him [2]. Pedersen found that the cavities of crown ethers, depending on their size, are capable of forming stable complexes with chemically similar metal ions. The complexation properties of crown ethers can be selectively fine-tuned by varying the size of the polyether macro-ring and by using different heteroatoms. By incorporating tricyclic heteroaromatic moieties (e.g. acridone unit), the conformational rigidity can be increased (Figure 1 C), which results in enhanced selectivity. Furthermore, the aromatic ring system is capable of forming π–π and cation–π interactions, which may also increase the complex stability. In addition, because of their fluoro- and chromogenic properties, the complexation ability of crown ethers containing an acridone unit can be evaluated by UV-vis and fluorescence spectroscopies.
The research goal, open questions
The development of ion sensors is of great importance because of their wide range of potential applications (pharmaceutical, pesticide and cosmetics industries). Using these substances, the detection of water contaminant metal ions becomes possible, the quantity of the contaminants can also be measured [3]. Therefore, our aim was to synthesize 18-crown-6 ether-based sensor molecules containing an acridone unit and also to study their complexation ability by UV-vis spectroscopy [4]. It was also our intention to reveal the mechanism of cation complexation using single-crystal X-ray diffraction (XRD) [5]. The enantiomers of optically active compounds may possess significantly different pharmacological activities, therefore, the precise determination of the enantiomeric ratio of these substances is of great significance. One of the most commonly used methods is high performance liquid chromatography (HPLC) on chiral stationary phases (CSP). The purpose of our research was to develop several acridino- and pyridino-18-crown-6 ether-based CSPs and evaluated their enantiomeric recognition abilities by HPLC method at Gedeon Richter Plc. [6−9] In order to better understand the nature of the enantiomeric discrimination ability, we have prepared both diastereomeric salts of an acridino-18-crown-6 ether and the enantiomers of the biologically important 1-(1-naphthyl)ethylamine for XRD measurements [10].
Methodology
The synthetic work was carried out using the methods of preparative organic chemistry. The progress of the reactions was followed by thin layer chromatography. The crude products were purified by column chromatography, recrystallization and trituration. Purity of the compounds was determined by thin layer chromatography, measuring the melting points and optical rotations. Structures of the products were determined using spectroscopic methods (IR,
1H-,13C-NMR and MS) and elemental analysis. Complexation properties of the sensors towards metal ions were studied by UV-vis spectroscopy in acetonitrile. UV-vis spectroscopy is based on the phenomenon that compounds with particular functional (chromophore) groups absorb light (wavelength range 10–780 nm) and a characteristic absorbance-wavelength spectrum can be recorded. If the sensor forms a complex with a metal ion, the absorbance spectrum of the complex changes accordingly and it may shift compared to the free sensor. Complex stability constants were determined by numerical methods and by global nonlinear regression analysis using ReactLab Equilibria™ program.
The enantiomeric separation ability of the novel selector molecules was studied by high performance liquid chromatography. The selector was first bound to silica gel support by covalent bonds, then a suspension was made of it and it was filled into an empty HPLC column at 500 bar pressure (slurry packing method). The covalent bounding allows the selector to be reused and it ensures the selectivity of the macro-cycle during complexation. Using continuous flow chemistry, a novel method has been elaborated for the preparation of chiral HPLC columns, by continuously recirculating the solution of a properly substituted crown ether through a HPLC column containing blank silica gel, under elevated pressure and high temperature. This procedure ensures covalent bond formation between the silica gel support and the crown ether. The novel columns were tested by HPLC using polar solvents (reverse phase method). Component separation is based upon the fact that the migration rate of individual compounds differs in certain circumstances, therefore, they leave the column at a different time. In chiral chromatography the selector molecule forms complexes with the enantiomers of chiral molecules with different complex stability constants, thus the separation becomes possible.
In order to reveal the mechanism of the ion and enantiomeric discrimination processes, few hundred µm-dimensional single crystals were formed by using different solvents from the appropriate crown ether hosts and selected guest molecules, and they were studied by single crystal X-ray diffraction (XRD). The method is based on that X-rays passing through the crystal suffer diffraction because their wavelength range (10-10 – 10-13 m) is comparable to the distances between the appropriate lattice planes in the crystal. Using this method, the spatial arrangement of the atoms of an unknown crystal can be determined. From the data the presence of secondary interactions, formed during the complexation processes, can be revealed.
Results
Part of my PhD work consisted of the synthesis of acridono-18-crown-6 ether-based sensor molecules. The synthesis of sensors
1 and 2 (Figure 2 A) and their nine precursors has been accomplished [4]. The cation recognition ability of the sensors toward nine metal ions was studied by UV-vis spectroscopy. We demonstrated the selective binding of lead(II) ions by sensor
1 [4] (Figure 2 B). To further study the complex, suitable single crystals for X-ray analysis were obtained from lead(II) perchlorate and sensor
1 [5]. The XRD measurements were carried out in collaboration with the Institute of Enzymology of the Hungarian Academy of Sciences, and with the Department of Applied Biotechnology and Food Science of BUTE.
The measurements showed that the crown ether is present in two tautomeric forms, which are in dynamic equilibrium with each other. One of the monomers remained in the acridone form and complexes a water molecule, but the other monomer shifts to the hydroxy-acridine form and complexes a lead(II) ion (Figure 2 C, two perchlorate ions are also present in the crystal).
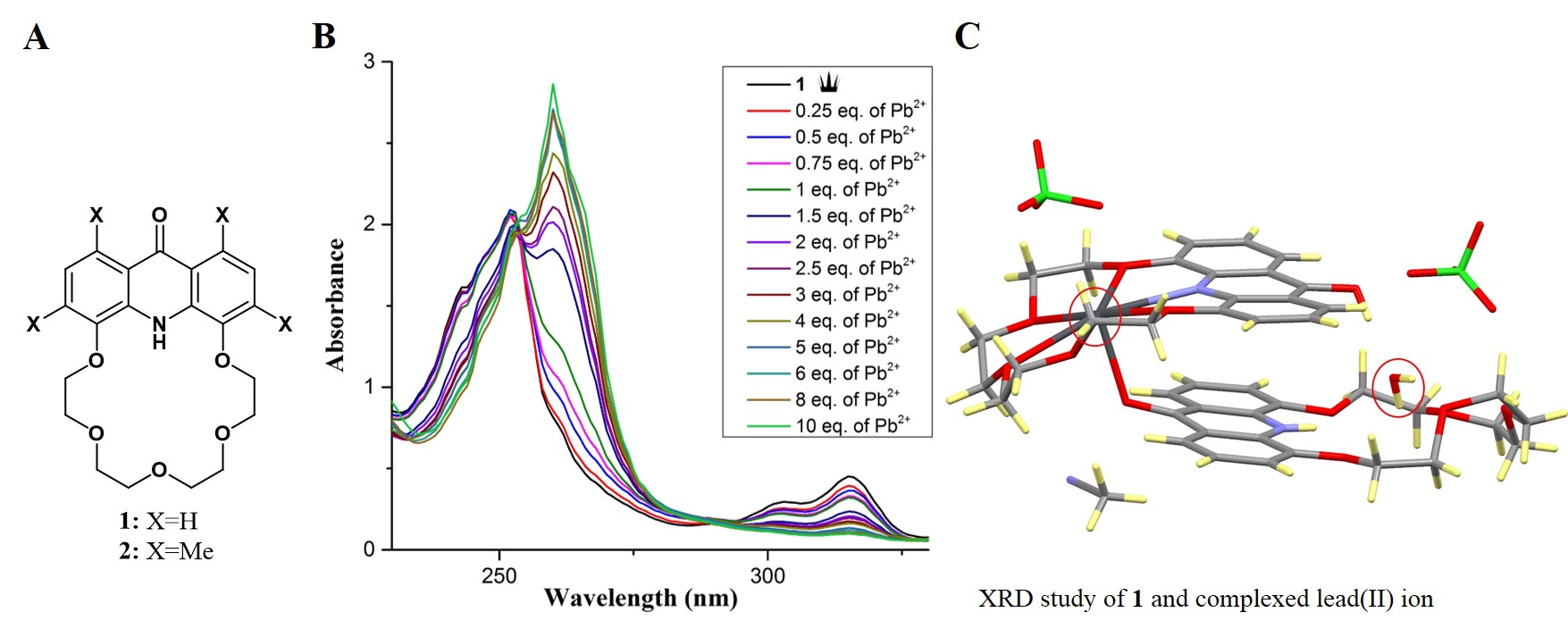
Figure 2
The other part of my work covered the preparation of chiral stationary phases. I took part in the evaluation of a pyridino-18-crown-6 ether-based CSP [6]. I have also successfully synthesized five acridino-crown ether based selector molecules and new CSPs were prepared from (R,R)-3 and (R,R)-4 (Figure 3 A, B) [7−9].
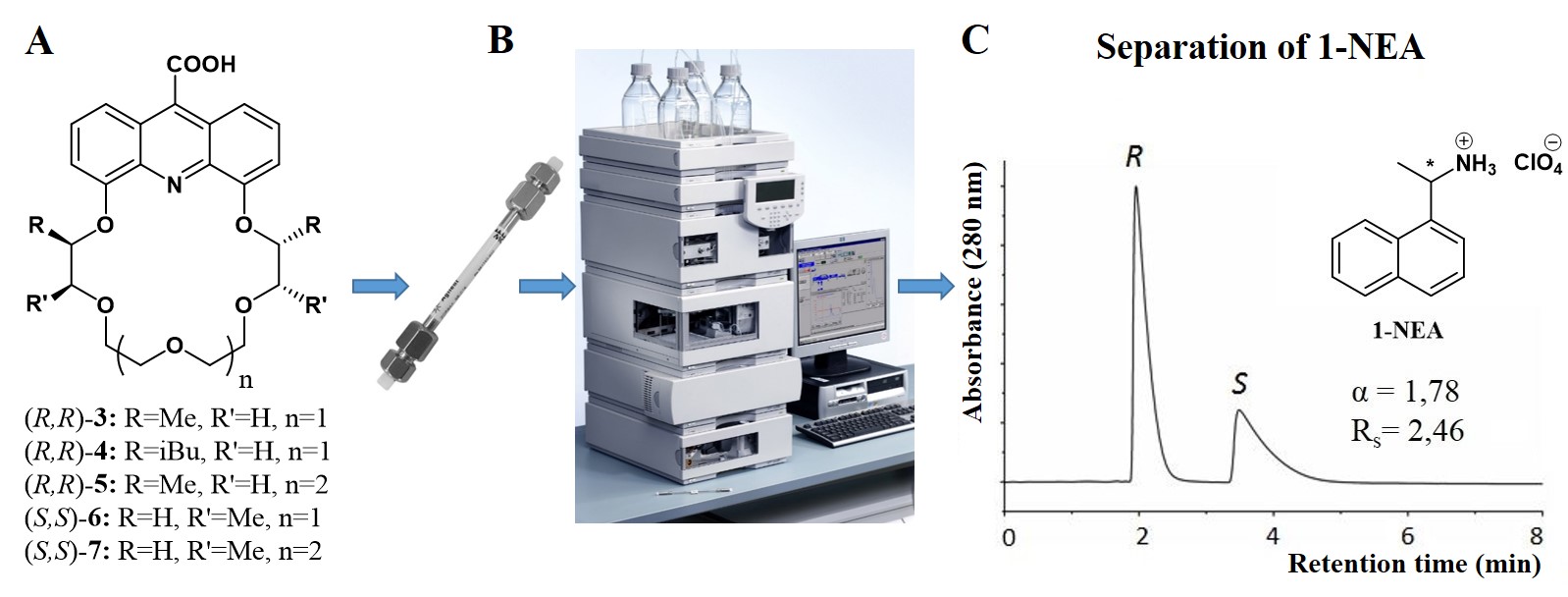
Figure 3
The enantiomeric separation ability of the CSPs toward protonated primary amines was studied at Gedeon Richter Plc. Biologically important amines were chosen as test compounds, for example 1-(1-naphthyl)ethylamine [1-NEA,
Figure 3 C] has several applications: it can be applied as a heterogeneous (two or more substances present in different phases, e.g. solid-liquid) catalyst, some of its derivatives also possess local anesthetic and anti-arrhythmic activities, therefore the purity of this substance is of great importance.
Baseline separations and high selectivities were achieved for the studied compounds (Figure 3 C). A novel method has also been elaborated for the preparation of CSPs, (R,R)-3 was bound to solid support by flow chemistry method [8]. In order to study the mechanism of the enantiomeric discrimination in the HPLC column, I have prepared suitable crystals from (R,R)-8 [an analogue of (R,R)-3)] and both enantiomers of 1-NEA (Figure 4). As in the case of the HPLC column, heterochiral preference was observed: (R,R)-8 formed more stable complex with (S)-1-NEA (Figure 4) [10].
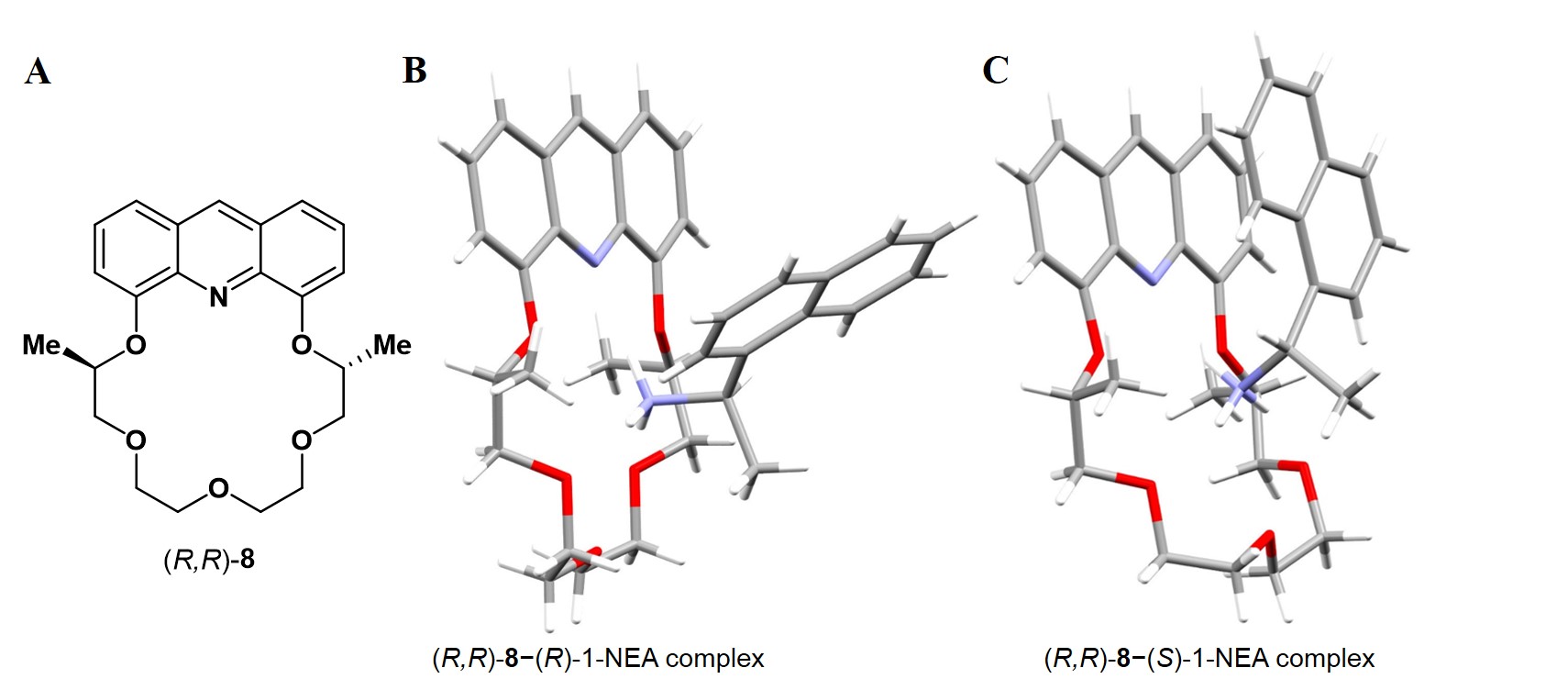
Figure 4
Expected impact and further research
During my work I synthesized acridono-crown ether-based sensor and acridino-crown ether-based selector molecules. The results are summarized in five internationally recognized journals, two other publications are being submitted. The research is part of an international (CRP/HUN14-01 ICGEB Research Grant) and three Hungarian (OTKA K112289, K109486, TÁMOP-4.2.1/B-09/1/KMR-2010-0002) projects. Our further aim is to prepare derivatives of sensor 1, which contain lipophilic side chains in order to prepare ion-selective electrodes. Thus, apart from the detection of lead(II) ions in wastewater, the measurement of its quantity may also be possible. Our other objectives include the preparation of new chiral stationary phases from (R,R)-5, (S,S)-6 and (S,S)-7 by flow chemistry method.
Publications, references, links
The list of related publications.
[A] Németh T, Kormos A, Tóth T, Balogh Gy T, Huszthy P
Monatshefte für Chemie 2015, 146: (8) pp. 1291–1297.
[B] Németh T, Golcs Á, Leveles I, Tóth T, Vértessy B G, Huszthy P
Structural Chemistry 2015, 26: (5) pp. 1467–1471.
[C] Lévai S, Németh T, Fődi T, Kupai J, Tóth T, Huszthy P, Balogh Gy T
Journal of Pharmaceutical and Biomedical Analysis 2015, 115: pp. 192–195.
[D] Németh T, Lévai S, Kormos A, Kupai J, Tóth T, Balogh Gy T, Huszthy P
Chirality 2014, 26:(10) pp. 651–654.
[E] Németh T, Lévai S, Fődi T, Kupai J, Túrós Gy, Tóth T, Huszthy P, Balogh Gy T
Journal of Chromatographic Science 2015, 53:(3) pp. 431–435.
[F] Németh T, Dargó G, Petró J L, Krámos B, Béni Z, Nagy J, Balogh Gy T, Huszthy P,
Tóth T in preparation for Journal of Pharmaceutical and Biomedical Analysis
[G] Tóth T, Németh T, Leveles I, Vértessy B G, Huszthy P in preparation for
Structural Chemistry
Links
References.
[1] Steed J W, Atwood J L, Supramolecular Chemistry
2009, Wiley, 2nd edition.
[2] Pedersen C J Journal of the American Chemical Society 1967, 89, 2495–2496.
[3] Chemosensors of Ion and Molecule Recognition; Desvergne J P, Czarnik A W, Eds.
NATO ASI Series C; Kluwer: Dordrecht, The Netherlands,
1997, Vol. 492.
[4] Németh T, Kormos A, Tóth T, Balogh Gy T, Huszthy P
Monatshefte für Chemie
2015, 146: (8) pp. 1291–1297.
[5] Németh T, Golcs Á, Leveles I, Tóth T, Vértessy B G, Huszthy P
Structural Chemistry 2015, 26: (5) pp. 1467–1471.
[6] Lévai S, Németh T, Fődi T, Kupai J, Tóth T, Huszthy P, Balogh Gy T
Journal of Pharmaceutical and Biomedical Analysis 2015, 115: pp. 192–195.
[7] Németh T, Lévai S, Kormos A, Kupai J, Tóth T, Balogh Gy T, Huszthy P
Chirality 2014, 26:(10) pp. 651–654.
[8] Németh T, Lévai S, Fődi T, Kupai J, Túrós Gy, Tóth T, Huszthy P, Balogh Gy T
Journal of Chromatographic Science 2015, 53:(3) pp. 431–435.
[9] Németh T, Dargó G, Petró J L, Krámos B, Béni Z, Nagy J, Balogh Gy T, Huszthy P,
Tóth T in preparation for Journal of Pharmaceutical and Biomedical Analysis
[10] Tóth T, Németh T, Leveles I, Vértessy B G, Huszthy P in preparation for
Structural Chemistry
