|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Department of Chemistry and Technology/Bioorganic Chemistry Research Group
Supervisor: Dr. Balogh-Weiser Diána
Development of a biomimetic system and its application for the preparation of drug metabolites
Introducing the research area
The transformations of drug candidate molecules in the human body (metabolism) are usually investigated using living organism-based models and cell based methods prepared from living organisms. Biomimetic systems, which can mimic the processes in living organisms, can offer robust alternative methods to prepare metabolites eliminating many disadvantages of the biological methods.
Brief introduction of the research place
I do my research at the Department of Organic Chemistry and Technology, BUTE in the Bioorganic Chemistry Research Group. Our research group has a long tradition in applying biocatalytic transformations in batch and continuous flow systems. Our research group works together with the Soft Matters Research Group, BUTE on developing supporters for biocatalysts and with the Biomimetic Technology Research Group, BUTE on developing immobilized biomimetic catalysts.
History and context of the research
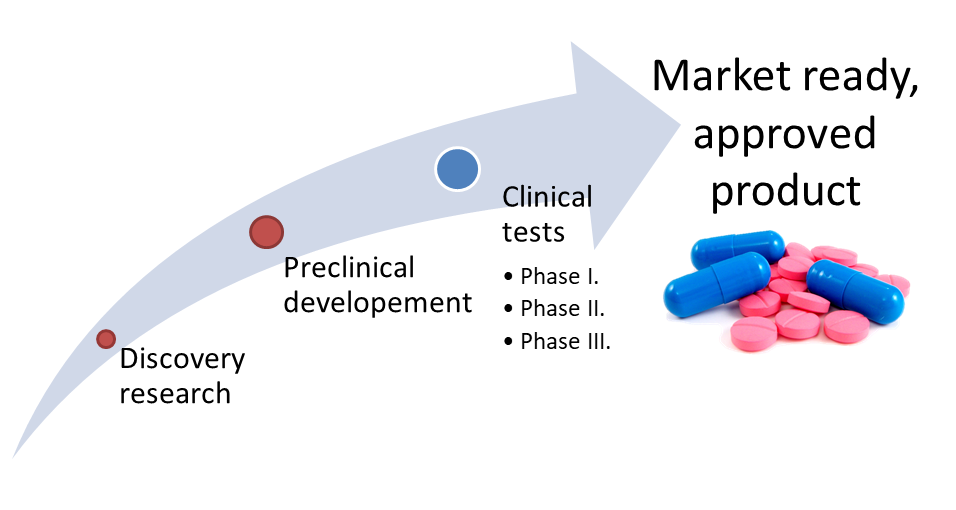
Figure 1: The route of drug development1
During the preclinical development (Figure 1.) of new drug candidate molecules (new chemical or biological entities - NCEs or NBEs) it is important to investigate their transformations in the human body (metabolism), how the pharmacology2 of these new compounds is changed (pharmacodynamics3 - effectiveness, pharmacokinetics4 - the fate of the drug in the human body). During metabolism main effect (prodrug approach), side effect or possibly toxic effect may occur. [1] In preclinical tests, living organisms may be used (animal models) or cell-based methods prepared from living organisms (Figure 2.).
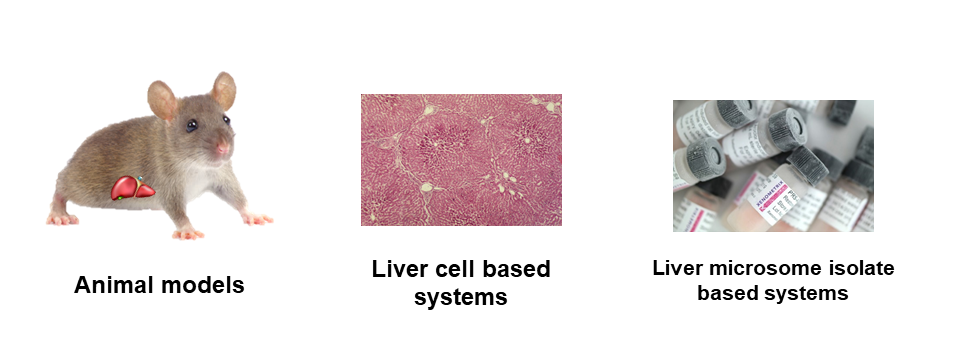
Figure 2: Examples of the biologic systems used in the metabolism studies
These biological methods are complicated, limited (only quantitative information can be obtained), relatively expensive and ethical issues can also be raised. [2] The application of biomimetic systems can offer a great alternative. Biomimetic systems can model the drug metabolism in the body. Synthetic metalloporphyrins5 are one promising group of biomimetic catalysts. The use of the synthetic metalloporphyrins as biomimetic catalysts is based on their structural similarity to the protoporphyrin-IX unit at the active site of heme6-containing proteins (CYP4507). [3] (Figure 3)

Figure 3: Comparing the general structure of Protoporphyrin IX and synthetic metalloporphyrins
Groves and his coworkers used synthetic metalloporphyrins as catalyst for the first time in the hydroxylation8 and epoxidation9 reactions of alkanes10 and olefins11 in 1979. [4] Chauhan and his coworkers used metalloporphyrins for the first time in the early 90' to model the metabolism of drug molecules. They prepared the main metabolite of the non-steroid anti-inflammatory drug etodolac in a biomimetic reaction. [5]
The research goals, open questions
Authorities require the determination of the exact structure and biological effect of the metabolites formed in the human body. Metabolites are formed only in low concentrations in the currently used biological systems and the present complex biological matrix makes the analysis more difficult. Preparation of metabolites in higher amounts is usually only possible through the development of new synthetic routes, which can increase the duration and expenses of the drug development. With the use of synthetic metalloporphyrins both the qualitative analysis (no complex biological matrix, and metabolites are formed in higher concentration) and the preparation of metabolites in higher amounts can be achieved. The metabolite composition of the reaction mixture can be enhanced by the systematic change of the reaction conditions (temperature, properties of oxidizing agent and metalloporphyrin, pH), thus the composition can be shifted towards the higher amount of the desired metabolite. The favorable conditions can be determined by optimizing the biomimetic reaction. A disadvantage of using synthetic metalloporphyrins is their easy degradation (for example metalloporphyrins can form the so-called µ-oxo-dimers in alkaline conditions which can inactivate the catalyst [6]), therefore, the stability of the porphyrins should be improved. The volume achieved by a homogeneous reaction may limit the formation of metabolites in large volumes, which can be overcome by using more catalyst; however, this significantly increases the expenses of the synthesis. These problems can be solved by the immobilization of porphyrin on solid supporters. The immobilized porphyrin based catalyst systems can offer a unique opportunity for high-efficiency, continuous-flow reaction pathway. During my research I try to find a novel solution for these problems by developing immobilized catalytic systems.
Methods
The porphyrins and solid supporters used during my research are prepared by modern preparative organic chemistry methods. The purity of porphyrin is determined by offline ultraviolet and visible light spectrophotometry (UV-vis)12. The porphyrin is purified by preparative thin layer chromatography13. Solid supporters are synthesized and modified in a heterogeneous reaction under optimized conditions. The development of a system suitable for the production of metabolites consists of several sub-steps. In each case, the first step is to optimize the homogeneous biomimetic reaction based on the considerations described previously. The reaction mixtures are analyzed by high-performance liquid chromatography coupled mass spectrometry (HPLC-MS)14. The optimal reaction conditions are selected based on the metabolite profile of the reaction and the conversion efficiency of the starting compound. Reference experiments are performed in the absence of catalyst for all oxidizing agents to exclude cases of significant non-biomimetic transformation. Unless a larger amount of metabolite is required, the only purpose is to identify the exact structure of the formed metabolites. The formed metabolites are separated by preparative thin layer chromatography or preparative high-performance liquid chromatography (preparative HPLC)15. The determination of structures is performed by high-resolution mass spectrometry (HRMS)16 as well as nuclear magnetic resonance (NMR)17 measurements. If the goal is to prepare a larger amount of metabolites (effect study, other goal), the next step is the selection of a solid support for the immobilization of the chosen porphyrin in heterogeneous batch reactions. In this step, the activity of the catalyst is examined by HPLC-MS. In the subsequent step, the heterogeneous batch system found to be optimal is transferred into a continuous flow reaction. Rapid optimization of the continuous flow system is performed by in-line UV-vis method as well as Raman spectroscopy18. The reaction mixture is analyzed by HPLC-MS. The desired compound is separated by preparative HPLC. As a reference, mouse, rat and human liver microsomal studies19 of the drug compounds were performed. The microsome-treated reaction mixtures were analyzed by the HPLC-MS technique mentioned earlier. To analyze the reaction mixture flowing through a microfluidic chip, coupled HPLC-MS technique was applied.
Results
In our research group, biomimetic oxidation of several drug molecules has previously been performed under batch and continuous flow circumstances, but the effect of the reaction conditions has not been studied. In the early phases of my research, the effects of the pH of the reaction medium and the quality of the oxidizing agent on the biomimetic reaction were investigated using Amiodarone (a drug used in the treatment of arrhythmia) as a model compound. In homogeneous reaction condition three different oxidizing agents (hydrogen peroxide - H2O2, tert-butyl hydroperoxide - tBuOOH, sodium periodate - NaIO4) were studied and the pH of the reaction mixture was changed between acidic (pH = 2.7) and slightly alkaline (pH = 7.4). The structures of the previously unidentified metabolites of Amiodarone have been proved by systematically changing the conditions.
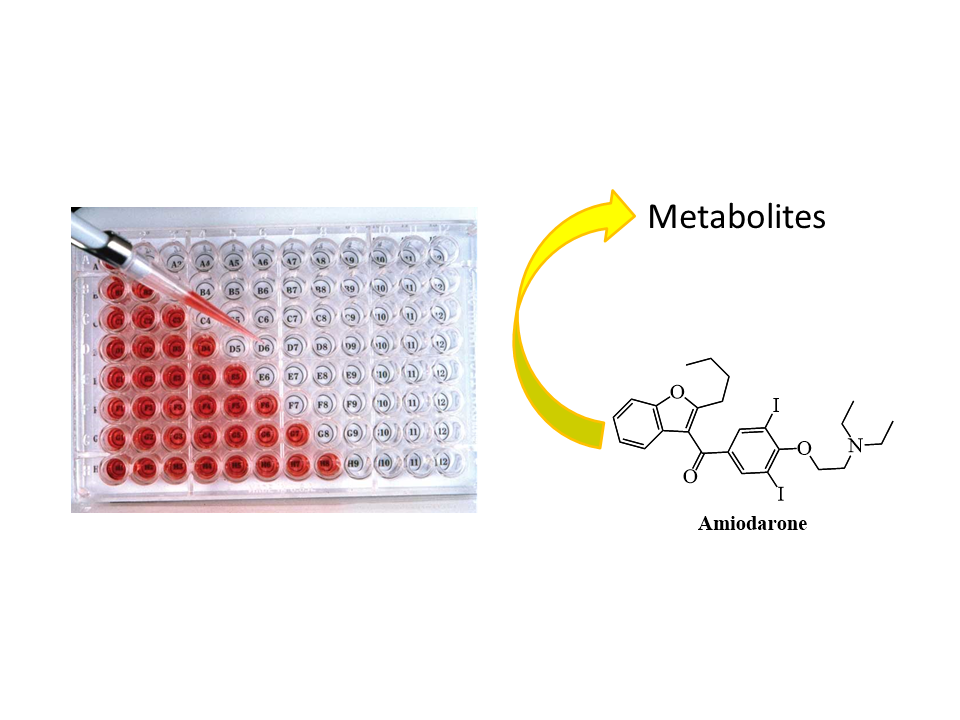
Figure 4: Demonstration of the homogeneous reaction
However, in a homogeneous condition, it was not possible to produce larger amounts of metabolites due to the short lifetime of the catalyst. After selecting the optimal reaction conditions (pH = 4.5, tBuOOH oxidizing agent), the biomimetic batch reaction had to be transferred to a continuous flow system, which was suitable for the production of larger amounts of metabolites. To achieve this goal, it was necessary to develop a suitable solid support, capable of binding the chosen porphyrin catalyst with good efficiency. A solid support suitable for our purposes was a mesoporous silica gel surface-modified with amino groups.
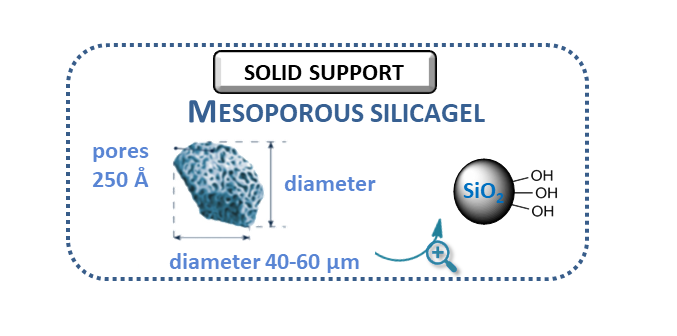
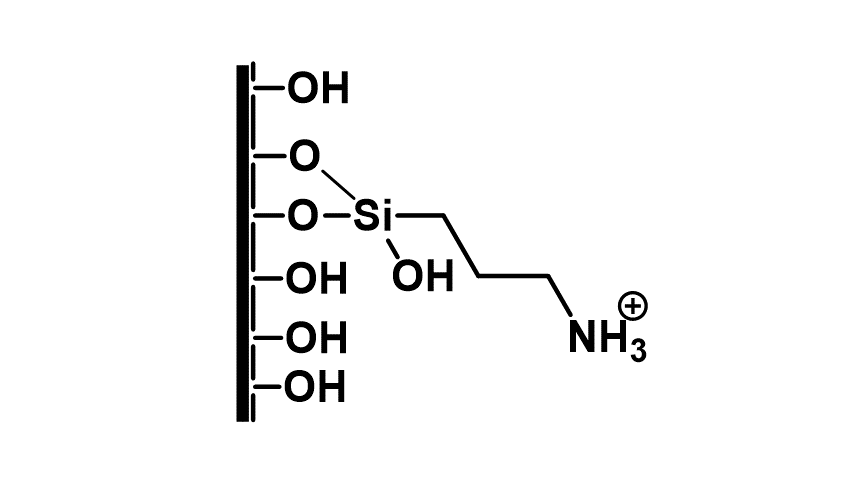
Figure 5: The properties of the mesoporous silica and the modified surface
This surface was capable of immobilizing the porphyrin catalyst by ionic interaction. The support was loaded into a tubular reactor, and then the catalyst was immobilized in continuous flow, which further improved the usability of the system (fast and easy preparation). The total amount of catalyst was bound on the surface of the support.
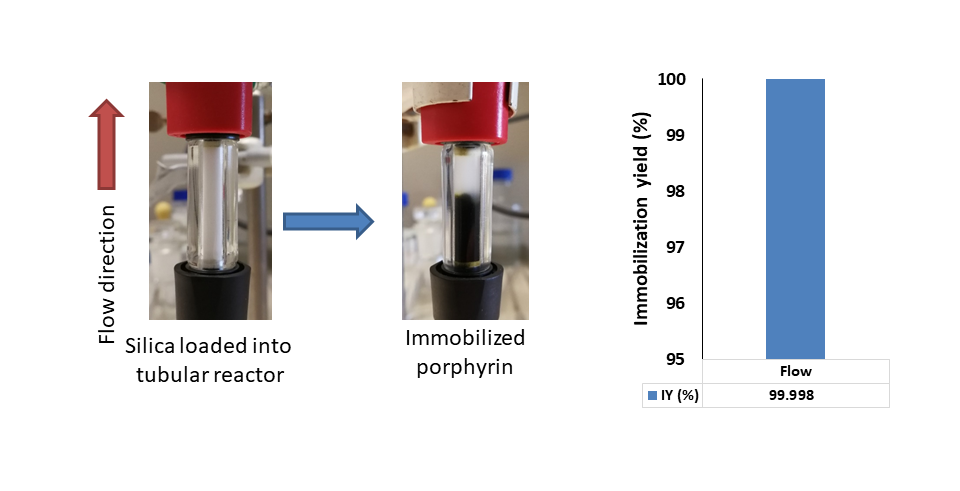
Figure 6: Immobilization of the porphyrin catalyst and the value of the immobilization yield (IY, %)
The effect of flow rate and the amount of oxidizing agent on the continuous-flow biomimetic reaction was investigated. Using the optimal conditions, the reaction proceeded for more than 50 hours without significant decrease in activity. Finally, the metabolite-producing ability of the available systems was compared. All three systems were characterized by volumetric productivity, which gives the mass of the product that can be produced per unit reactor volume per unit time. The continuous flow system we have developed is capable of an order of magnitude higher volumetric productivity than the previously developed continuous flow as well as the batch system.
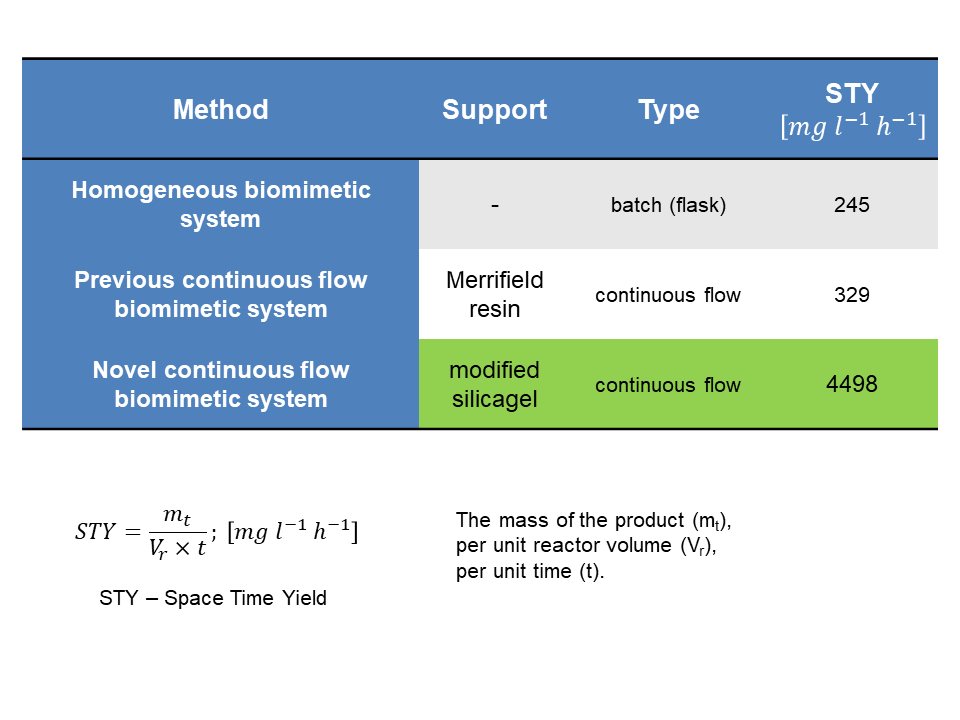
Figure 7: The space time yield (STY) and comparing the presented systems
Obtaining rapid analytical information is becoming increasingly important in drug discovery. For this purpose, the use of microfluidic chips that can provide information in a short time using a small amount of material can be advantageous. In the second half of my research, we aimed to develop a microfluidic chip reactor, which is capable of mimicking the liver based metabolism (liver-on-a-chip). A key issue here is the appropriate solid support that is suitable for simple, rapid trapping inside the microfluidic chip. The use of magnetic nanoparticles (MNPs) is expedient because, due to their magnetic properties, they can be trapped with a simple magnet inside the reactor. The chip we developed coupled with a suitable analytical technique (HPLC-MS) can produce and identify metabolites in up to 10 minutes.
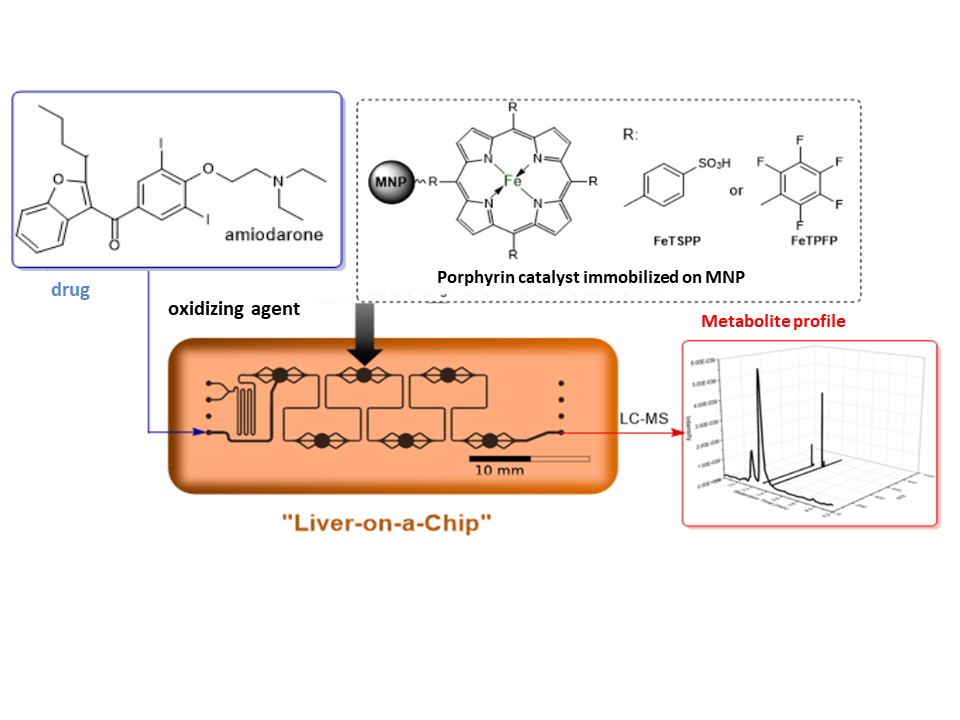
Figure 8: The general scheme of the microfluidic reaction
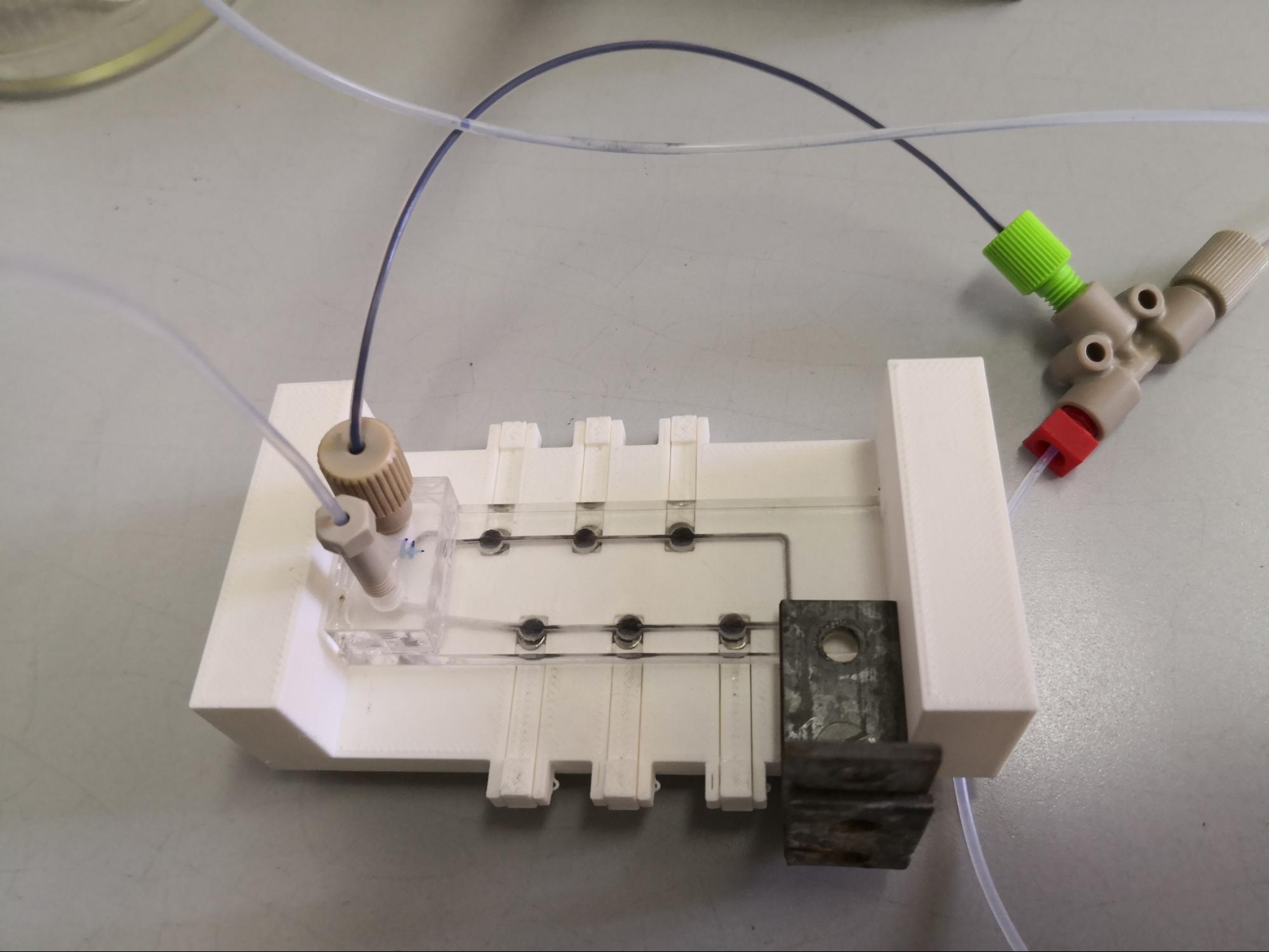
Figure 9: Microfluidic chip device in use
Expected impact and further research
In the course of my research, I developed a biomimetic system that can be used for the synthesis of metabolites, thus our system is capable of identifying the structure of metabolites that have not yet been identified or cannot be detected due to the biological matrix. The preparation of the metabolites required for the impact studies by the authorities becomes possible without developing new synthetic routes, which can speed up the drug development process. By producing and investigating new metabolites that are not formed in the body, we can broaden the available chemical field and make it possible to develop drugs with better properties.
In my further research, I aimed to investigate in more depth the factors influencing the biomimetic oxidation that can improve the selectivity of the reaction. In addition, I want to increase the stability of sensitive porphyrin catalyst, which can improve the productivity of the continuous flow reaction.
Publications, references, links
List of corresponding own publications
[S1] Decsi, B.; Krammer, R.; Hegedűs, K.; Ender, F.; Gyarmati, B.; Szilágyi, A.; Tőtős, R.; Katona, G.; Paizs, Cs.; Balogh, Gy., T.; Poppe, L.; Balogh-Weiser, D. Liver-on-a-Chip‒Magnetic Nanoparticle Bound Synthetic Metalloporphyrin-Catalyzed Biomimetic Oxidation of a Drug in a Magnechip Reactor. Micromachines 2019, 10, 668.
DOI: 10.3390/mi10100668
[S2] Fődi, T.; Ignácz, G.; Decsi, B.; Béni, Z.; Túrós, G.I.; Kupai, J.; Weiser, D.B.; Greiner, I.; Huszthy, P.; Balogh, Gy.T. Biomimetic Synthesis of Drug Metabolites in Batch and Continuous-Flow Reactors. Chem. - A Eur. J. 2018, 24, 9385–9392.
Table of links
1. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
2. https://en.wikipedia.org/wiki/Pharmacology
3. https://en.wikipedia.org/wiki/Pharmacodynamics
4. https://en.wikipedia.org/wiki/Pharmacokinetics
5. https://en.wikipedia.org/wiki/Porphine
6. https://en.wikipedia.org/wiki/Heme
7. https://en.wikipedia.org/wiki/Cytochrome_P450
8. https://en.wikipedia.org/wiki/Hydroxylation
9. https://en.wikipedia.org/wiki/Epoxide#Synthesis
10. https://en.wikipedia.org/wiki/Alkane
11. https://en.wikipedia.org/wiki/Alkene
12. https://en.wikipedia.org/wiki/Ultraviolet%E2%80%93visible_spectroscopy
13. https://en.wikipedia.org/wiki/Thin-layer_chromatography
14. https://en.wikipedia.org/wiki/Liquid_chromatography%E2%80%93mass_spectrometry
15. https://en.wikipedia.org/wiki/High-performance_liquid_chromatography
16. https://en.wikipedia.org/wiki/Mass_spectrometry
17. https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance
18. https://en.wikipedia.org/wiki/Raman_spectroscopy
19. https://en.wikipedia.org/wiki/Microsome
List of references
[1] Han, H., Amidon, G.L. Targeted prodrug design to optimize drug delivery. AAPS PharmSci 2, 48–58 (2000).
https://doi.org/10.1208/ps020106
[2] 1. Bernadou, J.; Meunier, B. Biomimetic Chemical Catalysts in the Oxidative Activation of Drugs. Adv. Synth. Catal. 2004, 346, 171–184.
DOI: 10.1002/adsc.200303191
[3] Lohmann, W., Karst, U. Biomimetic modeling of oxidative drug metabolism. Anal Bioanal Chem 391, 79–96 (2008).
10.1007/s00216-007-1794-x
[4] John T. Groves and Robert Quinn, J. Am. Chem. Soc. 1985 107 (20), 5790-5792
DOI: 10.1021/ja00306a029
[5] Chauhan SM, Kandadai SA, Sahoo B. Regioselective biomimetic oxidation of etodolac with iodosylbenzene catalyzed by halogenated and perhalogenated metalloporphyrins in dichloromethane. Chem Pharm Bull (Tokyo). 2001;49(10):1375-1376.
DOI:10.1248/cpb.49.1375
[6] Wolak, M. and van Eldik, R. (2007), Mechanistic Studies on Peroxide Activation by a Water‐Soluble Iron(III)–Porphyrin: Implications for OO Bond Activation in Aqueous and Nonaqueous Solvents. Chemistry – A European Journal, 13: 4873-4883.
DOI:10.1002/chem.200601148
