|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. Nagy Zsombor Kristóf
Development of integrated continuous pharmaceutical technologies
Introducing the research area
The pharmaceutical industry is the last among the large industrial sectors to use traditional batch technologies in the production to date. This obsolete concept has several disadvantages such as difficult scaling up or inflexible and slow production. Although many research papers have been published in the area of continuous pharmaceutical technologies, the vast majority of them examine individual steps, and technological integration essential for the wider industrial application has been involved sporadically. In this research work we examined the direct connection of different continuous pharmaceutical technologies and the new challenges arisen from the interconnection, covering the entire production chain from the starting materials to the final drug products.
Brief introduction of the research place
The research was carried out at the Department of Organic Chemistry and Technology of the Faculty of Chemical Technology and Biotechnology, under the supervision of Dr. György Marosi and Dr. Zsombor Kristóf Nagy. The FirePharma Research Group, led by them, has produced a number of publications of international interest in the field of continuous pharmaceutical technologies.
History and context of the research
Innovation in the pharmaceutical industry in recent decades has been limited to the research and development of new drug products, meanwhile the structure of the production has not changed in decades and still relies on outdated batchwise technologies. By replacing batch processes with continuous manufacturing, faster, cheaper and more flexible production can be developed with a significantly higher level of quality[1]. Recognizing this, the major pharmaceutical agencies began to encourage companies to develop and apply continuous technologies. As a result, extensive research is now being conducted from synthesis to final formulation. However, the vast majority of publications treat each manufacturing step separately. To take full advantage of continuous technologies, these separated processes need to be integrated. Based on the number of publications on the topic, it appears a big challenge to combine even two technological steps.
The ultimate aim of the continuous pharmaceutical process development would be the forming of end-to-end systems covering everything from the raw materials to the final dosage forms. There are only a few examples in literature for end-to-end manufacturing of drug products, where flow synthesis was connected to continuous formulation of either heat-mold tablets [2] or liquid dosage forms [3].
The research goals, open questions
The research was carried out in collaboration with foreign research groups and pharmaceutical companies. After surveying the current ‘state of the art’ related to integrated continuous pharmaceutical technologies, the main objectives of the experimental work could be set up. We aimed to develop continuous, end-to-end systems by implementing all traditional pharmaceutical manufacturing steps in a continuous manner, establishing direct connections between them, and examining both the new challenges arising from integration, and the long-term stability of the manufacturing process. Accordingly, flow synthesis, continuous crystallization and filtration, and continuous feeding and homogenization of crystal powder were the technological steps to be developed. In addition, we wanted to examine the feasibility of novel, intrinsically continuous but industrially not yet applied techniques, such as electrospinning for the direct processing of a flow reaction mixture. The final goal of the work was the production of final dosage forms: an innovative orally dissolving formulation made of the electrospun fibers, and the traditional compressed tablet although most widespread in industry not yet published in the literature.
Methods
The continuous flow synthesis of the selected model drug, acetylsalicylic acid was developed and optimized in PTFE microreactors, in which the liquids were fed with high-pressure precision pumps.
Crystallization of the reaction mixture was performed in a conventional stirred tank reactor with continuous input and product removal. Peristaltic pumps were used to feed the solutions, while the product slurry was discharged from the reactor using the transfer system of the continuous filtration device (Continuous Filtration Carousel, CFC), thus transferring the material directly to the next filtration step. Vacuum was applied in the CFC to transfer the suspension in small, 10 mL portions. The filtration process in the equipment took place in short, automated cycles.
The filtered active pharmaceutical ingredient and the tableting excipient, microcrystalline cellulose were continuously dosed into a twin-screw blender. The obtained powder blend was transferred by a conveyor belt to a tableting machine which continuously compressed it into conventional tablets.
In addition to the continuous crystallization, the reaction mixture of the flow synthesis was also processed by electrospinning. This technique required a dissolved polymer excipient beside the drug, which was introduced into the liquid stream during the synthesis. After the synthesis, high voltage was applied to a metal spinneret fixed to the end of the tubing. Due to the strong electrostatic field, nanofibers were formed from the viscous solution during the evaporation of the solvent. A continuous production equipment was developed in which the produced fibrous material was collected on a continuously moving carrier film. The double-layered strip formed in this way was conveyed further to an automated cutter mechanism to cut the product into smaller units ready for patient administration.
The developed processes and the resulting products were analyzed by several analytical methods: liquid chromatography, Raman and NIR spectroscopy, scanning electron microscopy, differential scanning calorimetry, mass spectrometry, nuclear magnetic resonance, gel permeation chromatography, X-ray powder diffraction, in vitro dissolution measurement, and laser diffraction for particle size measurement.
Results
The two-step synthesis of acetylsalicylic acid was developed in continuous flow reactors. During the selection of the reagents, solvents, and the catalyst the high volatility of the materials was an important aspect to obtain a reaction mixture suitable for processing by electrospinning. The two synthetic steps were optimized by design of experiment studies, thus excellent productivity and purity was achieved (>95%). By dissolving the optimized amount of polymer into the liquid stream, and by applying high voltage on the final reaction mixture, the produced acetylsalicylic acid was embedded into high quality nanofibers. We designed and built a continuous operation device for the continuous collection of the fibers on a moving carrier film, and the formed double-layered strip was cut into smaller dosage units (Fig. 1). The in vitro dissolution tests of the product showed instantaneous dissolution, thus the dosage form could be used as an orally disintegrating formulation.
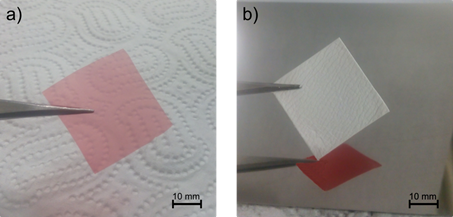
Fig. 1: 30 x 30 mm carrier film (a) and the electrospun fibers collected on its surface (b).
The stability of continuous production was tested in 8- and 24-hour long experiments, during which we were able to demonstrate the accuracy of the synthesis, fiber formation and fiber collection based on the purity of the products, the low residual solvent content of the fibers and the content uniformity of the products. A schematic drawing of the final implemented system is presented in Figure 2.
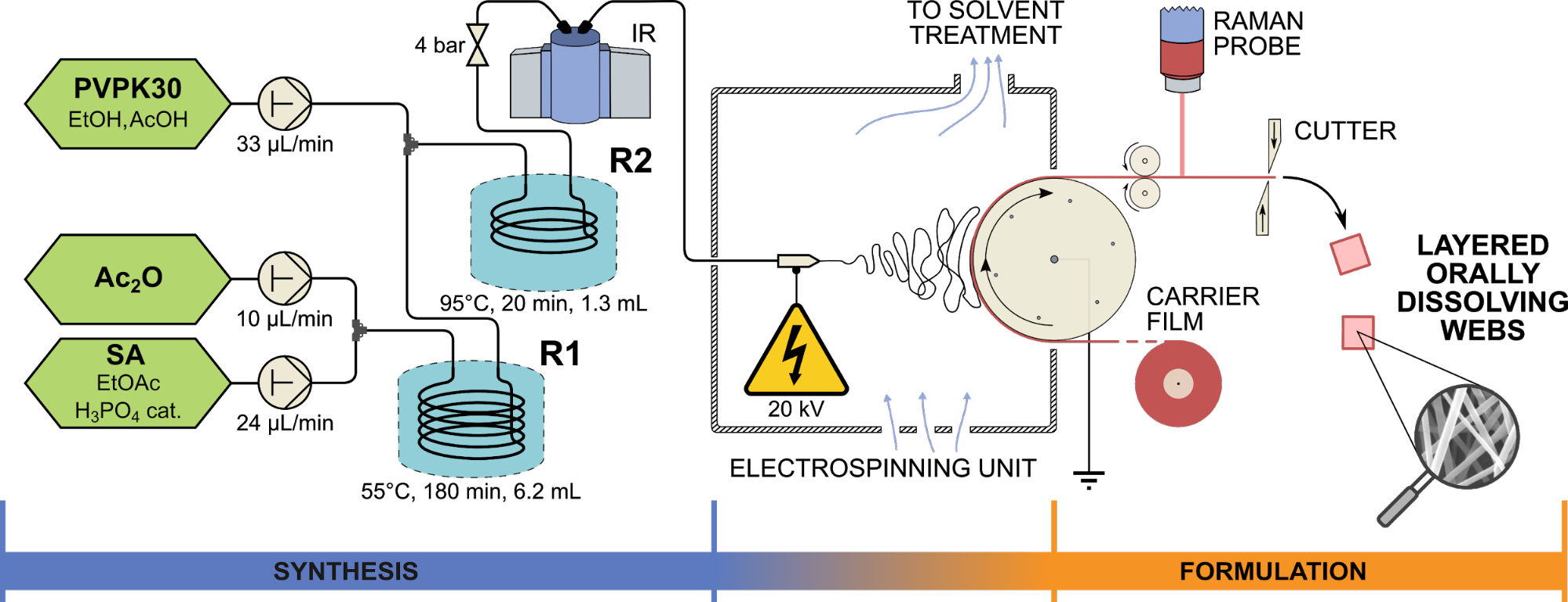
Fig. 2: Schematic drawing of the system developed for the production of ASA-loaded fibrous orally dissolving formulations based on electrospinning. R1 and R2 denote microreactors, IR is Bruker FTIR cell, and the Kaiser Raman PhAT probe was applied for monitoring.
The reaction mixture of the flow synthesis was also successfully processed by continuous crystallization, and the crystallized drug was directly transferred to a continuous filtration step. Heptane was used as an antisolvent in the continuous crystallization step. By modifying the reactor temperature and residence time, we could examine the change in the quality of the filtered product in terms of residual solvent content, yield, and particle size distribution. The results showed that the moisture content was influenced by both parameters (Fig. 3), while the crystal size and yield were influenced by the crystallizer temperature in the first place.
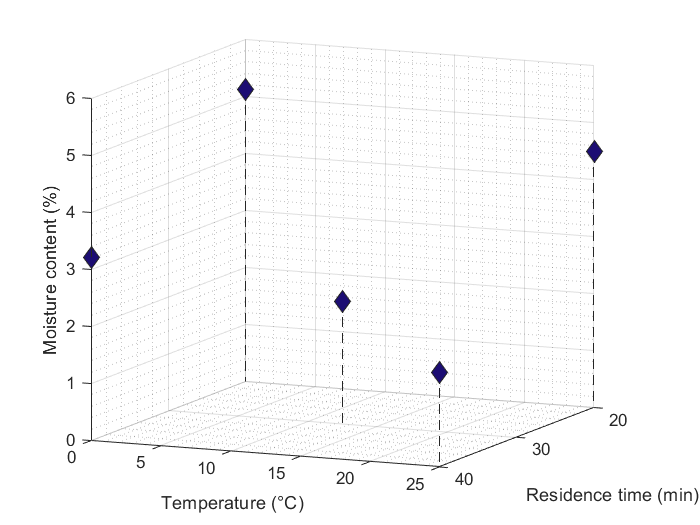
Fig. 3: The residual solvent content of the continuously filtered crystal product plotted against the temperature and the residence time of the continuous crystallizer.
An important observation was that the crystalline product produced with the continuous filtration device exhibited excellent flowing properties during each experiment, – a fundamental requirement for dosing the powder and for continuous homogenization (Fig. 4).

Fig. 4: Feeding of microcrystalline cellulose and acetylsalicylic acid into the hopper of the continuous blender (left); NIR probe mounted above the belt conveyor after blending (middle); continuous blender, belt conveyor and tableting machine integrated in one continuous manufacturing line (right).
These steps were monitored real-time with a near-infrared (NIR) spectroscopic probe, and the results showed that after a short start-up period the acetylsalicylic acid content of the powder blend reached the 20% target value with an acceptable deviation. The active pharmaceutical ingredient content of the tablets prepared in the next step was measured similarly with the NIR probe after production, resulting in a very similar average acetylsalicylic acid content and low deviation, which complied with the regulatory requirements (Fig. 5). Thus, the feasibility of the end-to-end continuous production of the most common dosage form, the compressed tablet was verified for the first time.
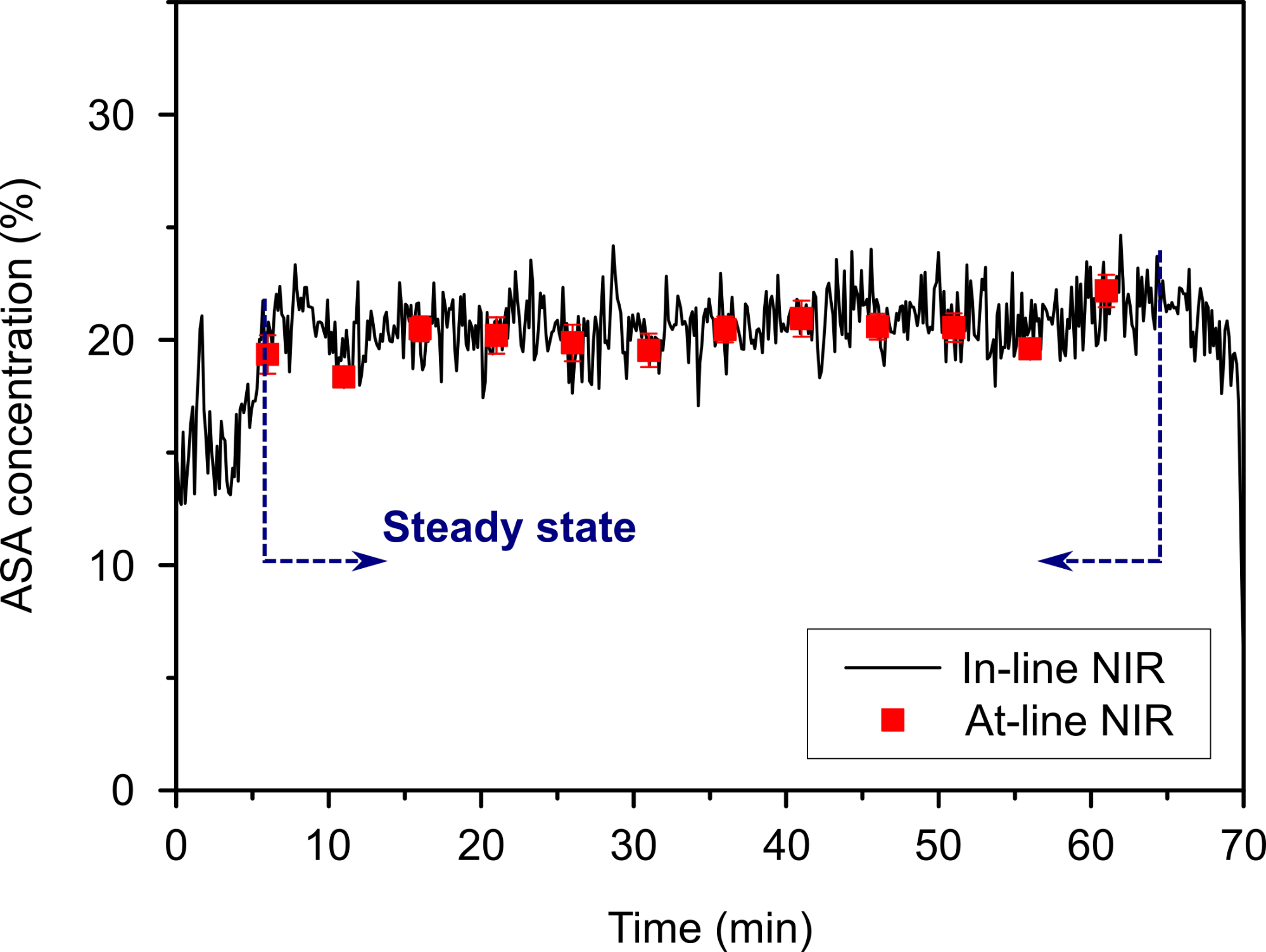
Fig. 5: Acetylsalicylic acid concentration in the powder blend measured by an in-line NIR probe after blending, and the result of at-line NIR analysis of the produced tablets from the steady state.
Expected impact and further research
In this work several continuous pharmaceutical processes were developed from drug substance to drug product manufacturing. Emphasis was put on the interconnection of these separate processes. The interaction of process parameters resulting from interconnection was evaluated as well. This unprecedented approach towards the development of continuous pharmaceutical processes can facilitate the industrial application of more and more continuous technologies and can contribute to the spread of continuous manufacturing in the pharmaceutical industry.
In the continuation of the research, we would like apply further active pharmaceutical ingredients in the developed processes, as well as to investigate further pharmaceutical manufacturing steps, such as continuous wet granulation and continuous drying, which areas are expected to draw significant attention.
List of corresponding own publications
Directly related publications
[I] A. Balogh, A. Domokos, B. Farkas, A. Farkas, Z. Rapi, D. Kiss, Z. Nyiri, Z. Eke, G. Szarka, R. Örkényi, B. Mátravölgyi, F. Faigl, G. Marosi, Zs. K. Nagy, Continuous end-to-end production of solid drug dosage forms: coupling flow synthesis and formulation by electrospinning, Chemical Engineering Journal, 350 (2018), 290–299.
IF: 8.355 C: 32
[II] A. Domokos, A. Balogh, D. Dénes, G. Nyerges, Z. Levente, B. Farkas, G. Marosi, Zs. K. Nagy, Continuous manufacturing of orally dissolving webs containing a poorly soluble drug via electrospinning, European Journal of Pharmaceutical Sciences, 130 (2019), 91–99.
IF: 3.616 C: 8
[III] Y. C. Liu, A. Domokos, S. Coleman, P. Firth, Z. K. Nagy, Development of continuous filtration in a novel continuous filtration carousel integrated with continuous crystallization, Organic Process Research & Development, 23, 12 (2019), 2655–2665
IF: 3.023 C: 1
[IV] A. Domokos, B. Nagy, M. Gyürkés, A. Farkas, K. Tacsi, H. Pataki, Y. C. Liu, A. Balogh, P. Firth, B. Szilágyi, G. Marosi, Z. K. Nagy, Zs. K. Nagy, End-to-end continuous manufacturing of conventional compressed tablets: from flow synthesis to tableting through integrated crystallization and filtration, International Journal of Pharmaceutics, 581 (2020), 119297.
IF: 4.845 C: 0
Indirectly related publications
[V] E. Borbás; B. Sinko, O. Tsinman, K. Tsinman; É. Kiserdei, B. Démuth, A. Balogh, B. Bodák, A. Domokos, G. Dargó, G. Balogh, Zs. K. Nagy, Investigation and mathematical description of the real driving force of passive transport of drug molecules from supersaturated solutions, Molecular Pharmaceutics, 13, 11 (2016), 3816–3826.
IF: 5.037 C: 38
[VI] A. Balogh, B. Farkas, Á. Pálvölgyi, A. Domokos, B. Démuth, G. Marosi, Zs. K. Nagy, Novel alternating current electrospinning of hydroxypropylmethylcellulose acetate succinate (HPMCAS) nanofibers for dissolution enhancement: the importance of solution conductivity, Journal of Pharmaceutical Sciences, 106, 6 (2017), 1634–1643.
IF: 3.075 C: 15
[VII] A. Balogh, B. Farkas, A. Domokos, A. Farkas, B. Démuth, E. Borbás, B. Nagy, G. Marosi, Zs. K. Nagy, Controlled-release solid dispersions of Eudragit® FS and poorly soluble spironolactone prepared by electrospinning and melt extrusion, European Polymer Journal, 95 (2017), 406–417.
IF: 3.741 C: 20
[VIII] B. Farkas, A. Balogh, A. Farkas, A. Domokos, E. Borbás, G. Marosi, Zs. K. Nagy, Medicated straws based on electrospun solid dispersions, Periodica Politechnica Chemical Engineering, 62, 3 (2018), 310–316.
IF: 1.382 C: 7
[IX] T. Casian, A. Farkas, K. Ilyés, B. Démuth, E. Borbás, L. Madarász, Z. Rapi, B. Farkas, A. Balogh, A. Domokos, G. Marosi, I. Tomuta, Zs. K. Nagy, Data fusion strategies for performance improvement of a Process Analytical Technology platform consisting of four instruments: An electrospinning case study, International Journal of Pharmaceutics, 567 (2019), 118473.
IF: 4.845 C: 3
[X] P. Vass, E. Szabó, A. Domokos, E. Hirsch, D. Galata, B. Farkas, B. Démuth, S. K. Anderson, T. Vigh, G. Verreck, G. Marosi, Zs: K. Nagy, Scale‐up of electrospinning technology: Applications in the pharmaceutical industry, WIREs Nanomedicine and Nanobiotechnology, 12, 4 (2019), e1611.
IF: 7.689 C: 6
[XI] L. A. Mészáros, D. L. Galata, L. Madarász, Á. Köte, K. Csorba, Á. Z. Dávid, A. Domokos, E. Szabó, B. Nagy, G. Marosi, A. Farkas, Zs. K. Nagy, Digital UV/VIS imaging: A rapid PAT tool for crushing strength, drug content and particle size distribution determination in tablets, International Journal of Pharmaceutics, 578 (2020), 119174.
IF: 4.845 C: 0
[XII] K. Tacsi, H. Pataki, A. Domokos, I. Csontos, I. Markovits, F. Farkas, Zs. K. Nagy, G. Marosi, Direct processing of a flow reaction mixture using continuous MSMPR crystallizer, Crystal Growth & Design, 20 (2020), 4433–4442.
IF: 4.089 C: 0
[XIII] P. Vass, E. Pantea, A. Domokos, E. Hirsch, J. Domján, Á. Németh, M. Molnár, Cs. Fehér, S. K. Andersen, T. Vigh, G. Verreck, I. Csontos, G. Marosi, Zs. K. Nagy, Electrospun Solid Formulation of Anaerobic Gut Microbiome Bacteria, AAPS PharmSciTech, 21, 214 (2020).
IF: 2.401 C: 0
List of references
[1]: S.L. Lee, T.F. O’Connor, X. Yang, C.N. Cruz, S. Chatterjee, R.D. Madurawe, C.M. V Moore, L.X. Yu, J. Woodcock, Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production, J. Pharm. Innov. 10 (2015) 191–199. doi:10.1007/s12247-015-9215-8
[2]: S. Mascia, P.L. Heider, H. Zhang, R. Lakerveld, B. Benyahia, P.I. Barton, R.D. Braatz, C.L. Cooney, J.M.B. Evans, T.F. Jamison, K.F. Jensen, A.S. Myerson, B.L. Trout, End-to-end continuous manufacturing of pharmaceuticals: Integrated synthesis, purification, and final dosage formation, Angew. Chemie. 125 (2013) 12585–12589. doi:10.1002/ange.201305429
[3]: A. Adamo, R.L. Beingessner, M. Behnam, J. Chen, T.F. Jamison, K.F. Jensen, J.-C.M. Monbaliu, A.S. Myerson, E.M. Revalor, D.R. Snead, T. Stelzer, N. Weeranoppanant, S.Y. Wong, P. Zhang, On-Demand Continuous-Flow Production of Pharmaceuticals in a Compact Reconfigurable System, Science. 352 (2016) 61–67. doi:10.1126/science.aaf1337
