|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Institute of Technical Physics and Material Science - Centre for Energy Research
Supervisor: Dr. Deák András
Investigating the surface modification and the self-assembly of gold nanoparticles
Introducing the research area
During my research, I investigated the binding of various thiol-containing ligands on gold nanorods’ surface utilising ensemble and single-particle measurement techniques. Understanding and tuning the structure of the boundary layer are essential for potential applications. Additionally, nanoparticle assemblies were prepared using properly surface modified gold nanoparticles as building blocks. Compared to the individual particles, the as-prepared structures feature special emerging optical properties.
Brief introduction of the research place
The research was performed at the campus of KFKI in the Institute of Technical Physics and Materials Science (MFA), a member of Centre for Energy Research (EK). The research of our group called Chemical Nanostructures Laboratory focuses mainly on the preparation and self-assembly of gold nanoparticles and the investigation of their optical properties. Our laboratory-built single particle spectroscopy instrument is unique in Hungary.
History and context of the research
The special optical properties of gold nanoparticles are due to the phenomenon of localised surface plasmon resonance. When noble metal nanoparticles are excited by an electromagnetic wave, the free electrons of the material start to oscillate as well. At a certain frequency in the visible wavelength range, the process becomes resonant, which is the so-called localised surface plasmon resonance. At this frequency, a pronounced peak can be found in the optical spectrum of a sphere-shaped gold nanoparticle. The resonance frequency is predominantly shape-dependent (e.g. two peaks can be observed in the spectrum of a gold nanorod) and also depends on the embedding medium and the composition of the boundary layer. [1] Furthermore, the particles’ optical near-field regions can interact with each other provided the particle-particle distance is sufficiently small. This results in a modified optical response due to coupling (also see surface enhanced Raman spectroscopy); the resonance peak shifts and broadens, even a new resonance peak can evolve. [2]
When particles of dissimilar shape are used to create a particle-assembly, a range of different optical features can develop. Deeper insight into the colloid interactions can facilitate the rational design of such particle assemblies. A possible approach for tuning the colloidal interactions can be the surface modification of the particles. Permanent or pH-responsive charge can be obtained by surface modification and aggregation can be avoided by appropriate use of macromolecules. Gold nanoparticles are typically modified by thiol group containing molecules due to the strong gold-sulfur interaction. [3] By changing certain experimental conditions (ionic strength, temperature, pH etc.) stabilising ligands become destabiliser. Hence the structure can be designed and the structure of the particle assembly controlled. [4]
The research goals, open questions
The aim of my research is to investigate the adsorption of thiol-containing molecules with different charge states (cysteamine, 3-mercaptopropionic acid) on hexadecyl(trimethyl)ammonium bromide (CTAB)-stabilised gold nanorods. In order to gain a deeper insight into the process, single particle experiments were also performed.
Furthermore, my goal is to prepare gold nanorods featuring surface heterogeneity; the tips of the nanorods are to be coated by a positively charged molecule called cysteamine, while the side region is to be covered by a neutral macromolecule called α-methoxy-ω-mercapto-polyethylene-glycol (m-PEG-SH).
Besides, the purpose of the work is to produce gold nanosphere-nanorod heterodimers using surface modified particles. I would like to optically characterise the developed structures and their re-arrangement possibilities.
Methods
CTAB-stabilised gold nanorods were prepared using a two-step wet-chemical synthesis procedure. [5] The success of the synthesis and of the surface modification was monitored by UV-Vis spectroscopy and electrophoretic mobility measurements. The narrow size distribution of the particles was verified utilising scanning electron microscopy as well. Typically, two peaks can be found in the spectrum of gold nanorods, since a transversal and a longitudinal mode evolve during the excitation. The shift of the longitudinal peak after the surface modification is attributed to the compositional change of the optical near-field region. In order to monitor the ligand exchange, electrophoretic mobility measurements were also performed, which provides information on the nanorod’s charge state as a whole. Using the aforementioned two approaches, the heterogeneous surface modification of nanorods can be verified. [6]
To gain a deeper insight into the composition and the structure of the surface layer of the rods, single particle spectroscopy measurements were carried out coupled with scanning probe measurements (Atomic Force Microscopy, Scanning Electron Microscopy). Using single particle spectroscopy, the spectrum of substrate-deposited individual particles and their assemblies can be obtained. The instrument is composed of a dark field microscope and an aberration corrected imaging spectrometer. During in situ measurements in aqueous medium, the microscope can be operated in transillumination mode as well. In such a case a flow-cell has to be assembled which was composed of two glass cover slips and a holder. After the optical excitation of the particles, the dark field image was projected onto the slit of the spectrometer by lenses. By proper positioning of the sample, the scattered light from the particle of interest will enter the spectrometer, where an optical grating resolves the spectrum according to wavelength and a CCD camera is used for detection. By fitting a Lorentzian function on the spectra of the single particles, the resonance wavelength position as well as the damping (FWHM – full width at half maximum) can be obtained. While the changes of the former parameter provide information about molecule adsorption at the tips, the increase of damping refers to the binding at the entire surface of the rod (like electrophoretic mobility measurements). A polariser can also be installed to separate the various oscillation directions of the scattered light.

Fig. 1 The single particle spectroscopy instrument used in our laboratory
Results
Investigation of thiol-ligands’ adsorption on gold nanorods [S1, S2]
The surface of CTAB-stabilised gold nanorods were modified using aqueous solutions of thiol-containing molecules (cysteamine, MPA) at various concentrations. The process was monitored by ensemble optical spectroscopy and electrophoretic mobility measurements.
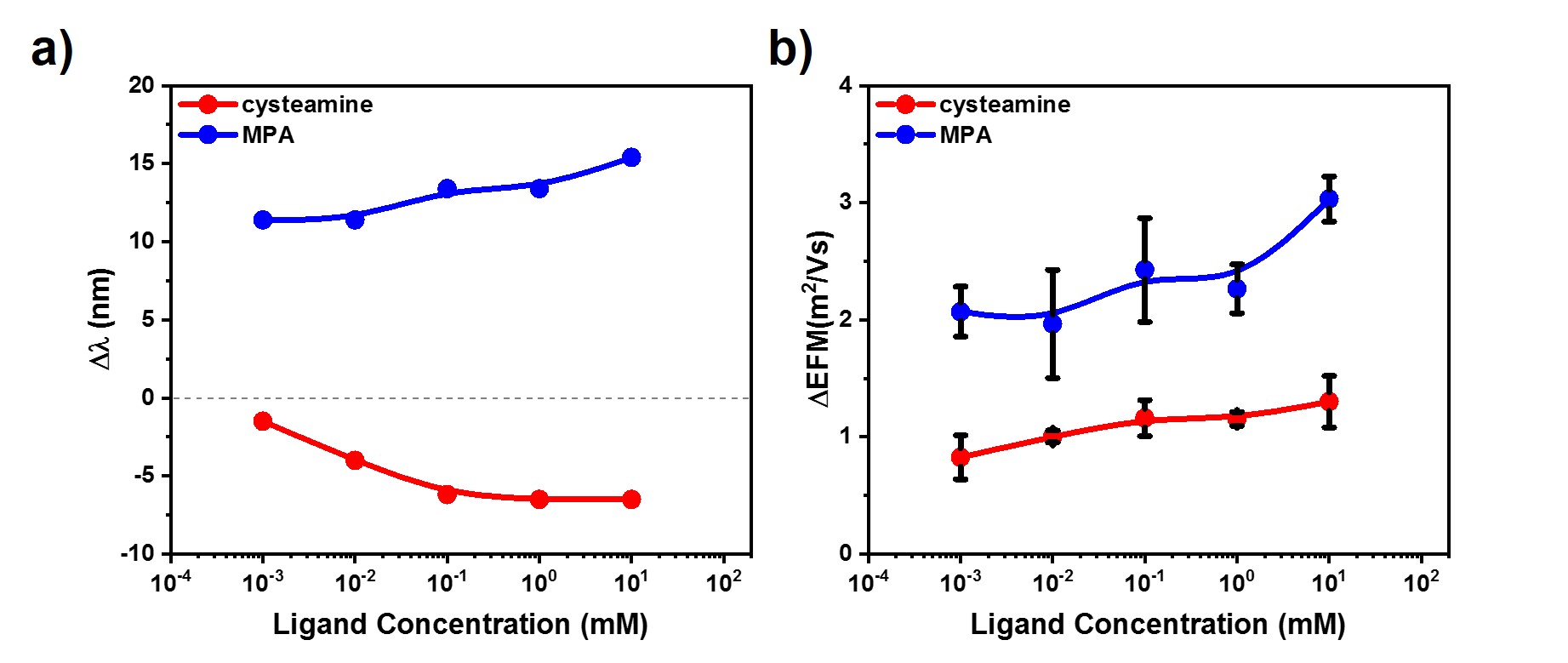
Fig. 2 The longitudinal resonance peak position (a) and electrophoretic mobility (b) changes during ensemble measurements
Fig. 2 shows that the direction of the longitudinal peak shift depends on the charge state of the given molecule. This can be related to the presence of the charged CTAB-layer on the surface, but the observed redshift for MPA might refer to aggregation as well. In order to rule out aggregation, single particle experiments were carried out. The averages obtained from experiments on individual nanorods can be seen in Fig. 3.
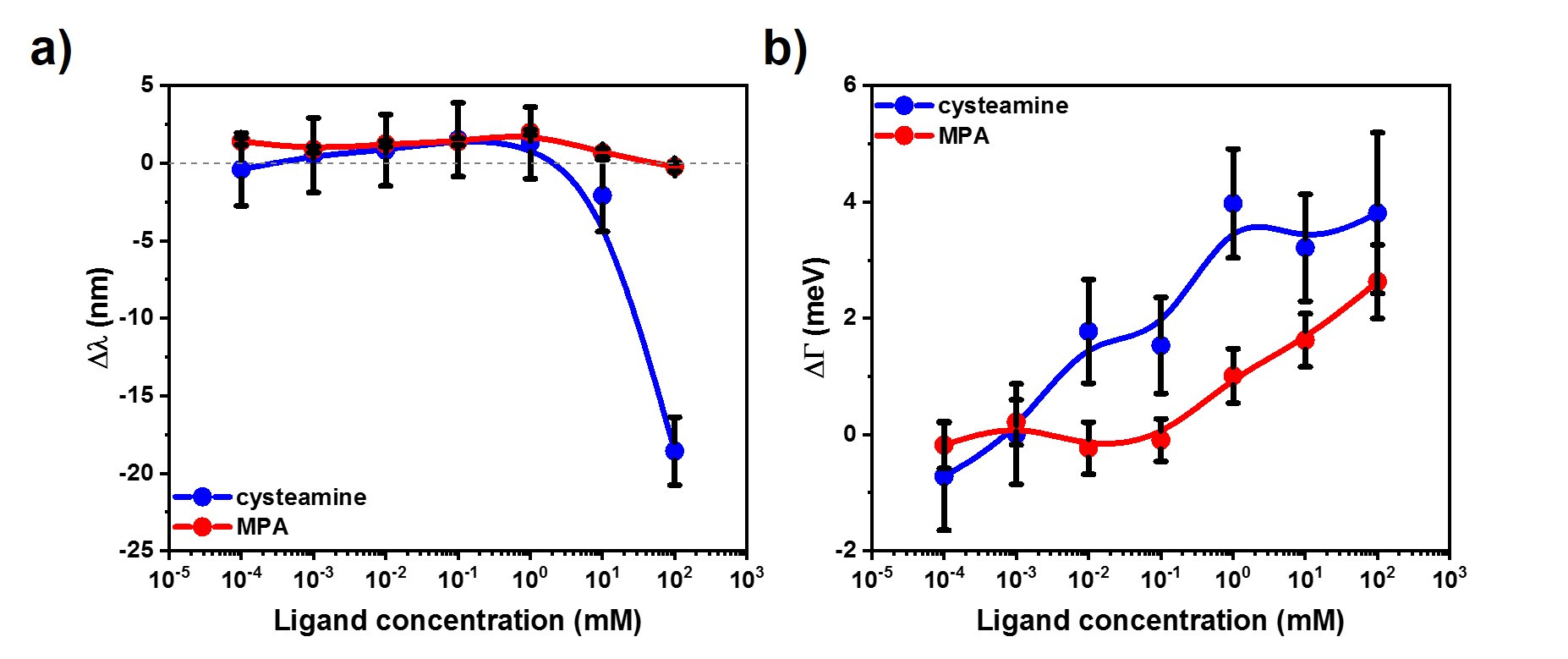
Fig. 3 The resonance peak position (a) and damping (b) changes obtained by single particle spectroscopy experiments
The experiments are in agreement with the ensemble measurements. The hypothesized mechanism suggests that the positively charged cysteamine can rapidly remove the similarly positively charged CTAB from the surface due to the strong gold-sulfur interaction. In the case of negatively charged 3-MPA the ligand exchange is a self-limiting process, since CTAB physically binds to the thiol-ligand layer as a consequence of electrostatic interactions. Above a critical MPA concentration the removal of CTAB begins which is indicated by the maximum of the FWHM curve. Increasing amount of redshift can be observed with increasing concentration of CTAB that corroborates the as-described mechanism (Fig. 4.a). In agreement with the theory, the FWHM curves asymptotically increase to a limiting value (Fig. 4.b).
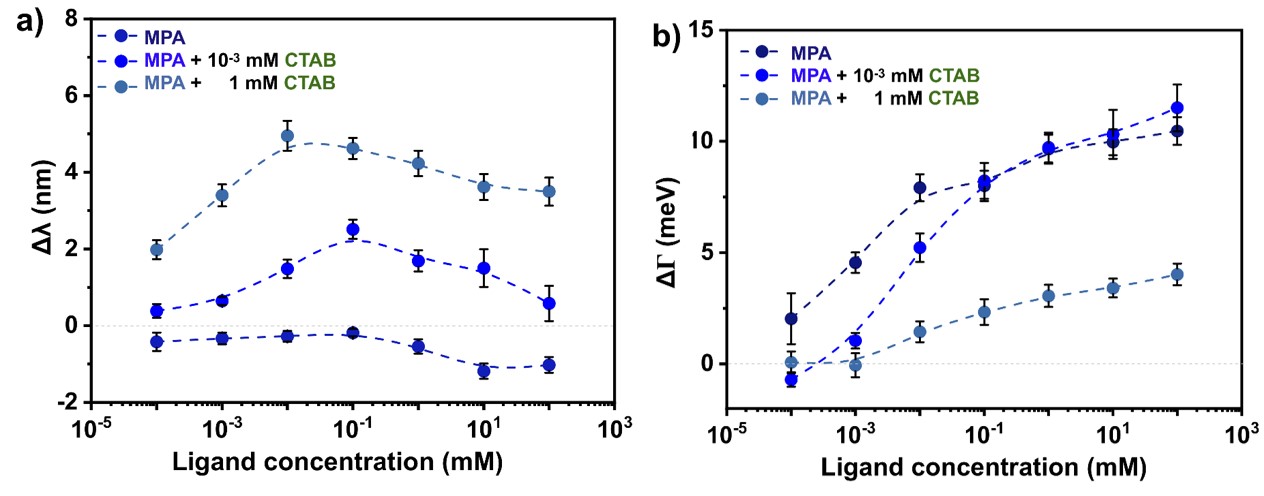
Fig. 4 The resonance peak position (a) and damping (b) changes with different CTAB concentration obtained by single particle spectroscopy experiments
In order to prepare gold nanorods with surface ligand heterogeneity a cysteamine concentration was used, where the blueshift of the longitudinal peak just reached its maximum value (e.g. in Fig. 2: ~0.1 mM). As a reference, samples nanorods covered by m-PEG-SH and (11-mercaptoundecyl)-N,N,N-trimethylammonium bromide (MTAB) were used. This latter was chosen because cysteamine alone cannot stabilise the gold nanorods. Furthermore, SEM micrographs were recorded in order to compare nanorods of approximately the same dimensions. AFM experiments were also performed on the same nanorods in order to investigate the structure of the boundary layer.
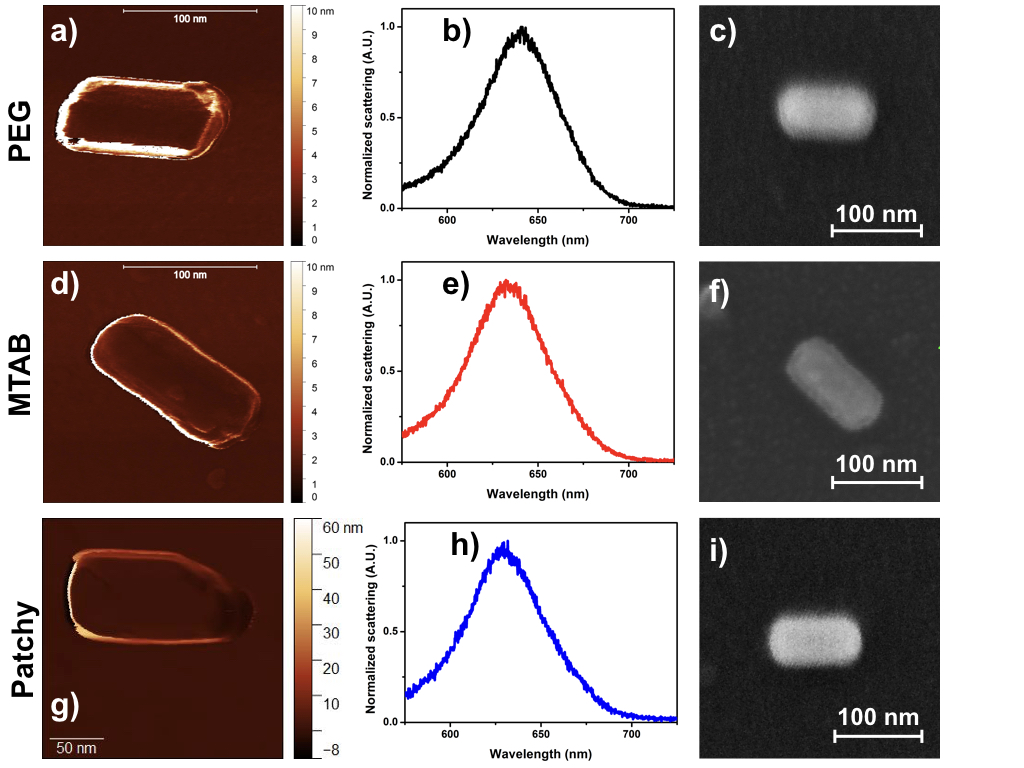
Fig. 5 AFM (a,d,g), SEM (c,f,i) micrographs and scattering spectra (b,e,h) of individual reference and patchy particles
In the AFM image of polymer-stabilised gold nanorod a few-nanometre thick layer can be observed, while the surface of CTAB-stabilised nanorod was covered by a much thinner coating. It is shown in Fig. 5. that a heterogeneous boundary layer can be created during the surface modification at a given cysteamine concentration.
Investigating the optical response of the gold nanosphere-nanorod heterodimers [S3]
Single particle spectroscopy experiments were carried out on sphere-rod heterodimers followed by an analysis of spatial arrangement of the heterodimers utilising scanning electron microscopy. Since the difference between the white-light optical spectrums of the two main arrangements was not significant, polarisation-resolved detection was performed (Fig. 6.)
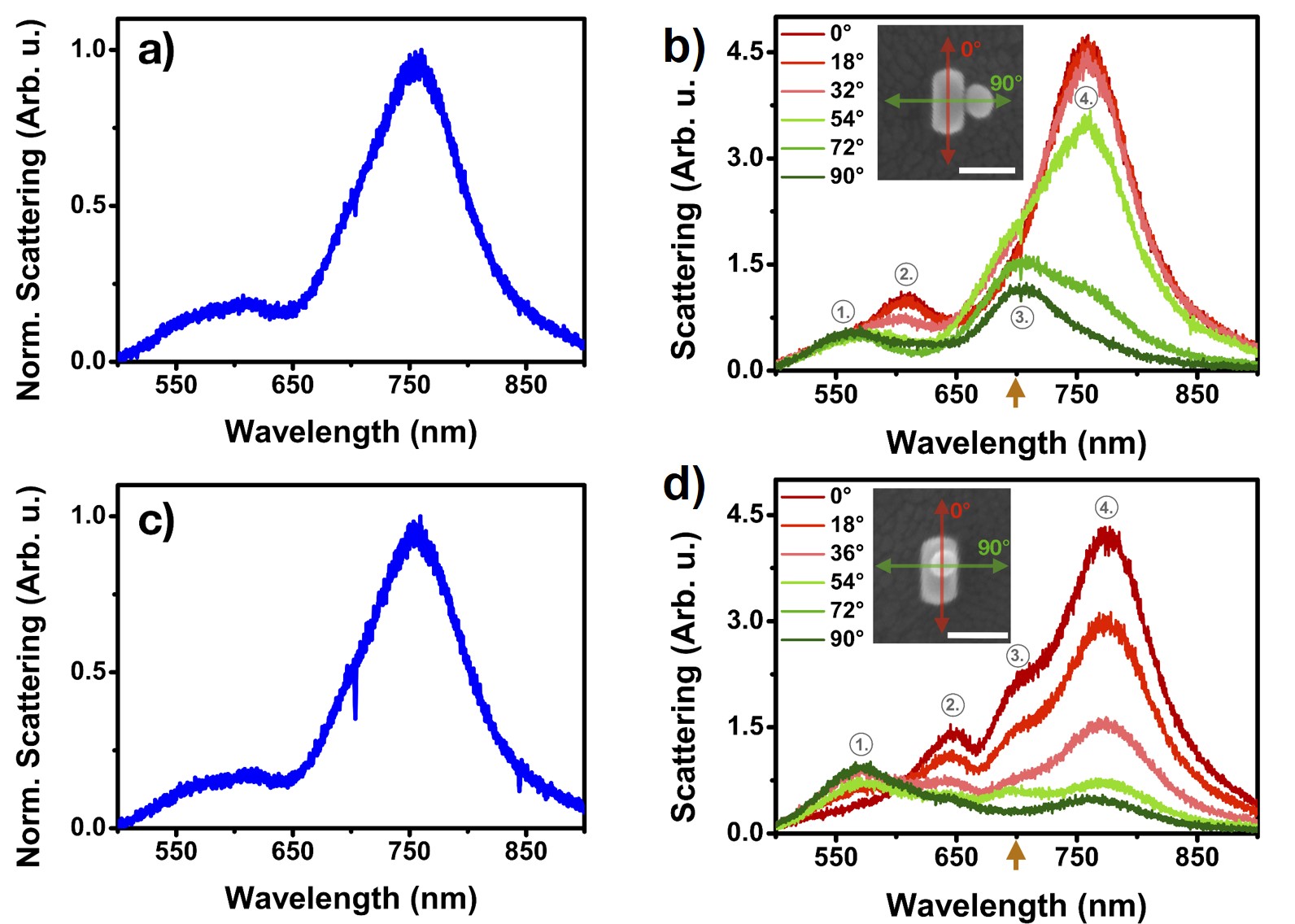
Fig. 6 The white-light and polarisation resolved spectra of two main nanosphere-nanorod arrangements
The scattering peaks observed at marked, specific wavelengths for the two arrangements in Fig. 6 change intensity differently with the analyser angle. My calculations confirmed that a coupling between the sphere dipolar/rod transversal modes can be observed at this wavelength. The structure dependent differences in the polarisation resolved spectra originate in the different spatial orientation of this coupled mode. Further experiments were carried out in order to investigate the possibility of any re-arrangement during drying of the self-assembled structures. The results indicated that in some cases the nanosphere located on the top of the nanorod can shift to the substrate level during the drying thanks to the immersion capillary forces.
Expected impact and further research
The optical properties of self-assembled structures can be more precisely designed via tuning the particle-particle distance. Our aim for the future is to determine the gap between sphere-shaped particles as a consequence of the changing experimental conditions (ionic strength, pH, the size of the ligands, temperature). The interparticle distance will be obtained analysing the scattering spectra of homodimers, based on the so-called plasmon ruler phenomenon. [7]
Publications, references, links
List of corresponding own publications
[S1] Szekrényes, D.P.; Kovács, D.; Zolnai, Zs.; Deák, A. Chemical Interface Damping as an Indicator for CTAB Replacement by Short-Chain Thiols on Gold Nanorods. The Journal of Physical Chemistry C 2020 (IF = 4.189)
[S2] Szekrényes, D. P.; Pothorszky, S.; Zámbó, D.; Osváth, Z.; Deák, A. Investigation of Patchiness on Tip-Selectively Surface-Modified Gold Nanorods. The Journal of Physical Chemistry C 2018, 122 (3), 1706–1710. (IF = 4.189)
[S3] Szekrényes, D. P.; Pothorszky, S.; Zámbó, D.; Deák, A. Detecting Spatial Rearrangement of Individual Gold Nanoparticle Heterodimers. Phys. Chem. Chem. Phys. 2019, 21 (19), 10146–10151. (IF = 3.430)
Other publications in this topic
Pothorszky, S.; Zámbó, D.; Szekrényes, D.; Hajnal, Z.; Deák, A. Detecting Patchy Nanoparticle Assembly at the Single-Particle Level. Nanoscale 2017, 9 (29), 10344–10349. (IF = 6.895)
Zolnai, Z.; Zámbó, D.; Osváth, Z.; Nagy, N.; Fried, M.; Németh, A.; Pothorszky, S.; Szekrényes, D. P.; Deák, A. Gold Nanorod Plasmon Resonance Damping Effects on a Nanopatterned Substrate. J. Phys. Chem. C 2018, 122 (43), 24941–24948. (IF = 4.198)
Albert, E.; Tegze, B.; Hajnal, Z.; Zámbó, D.; Szekrényes, D. P.; Deák, A.; Hórvölgyi, Z.; Nagy, N. Robust Contact Angle Determination for Needle-in-Drop Type Measurements. ACS Omega 2019, 4 (19), 18465–18471. (IF = 2.58)
Zámbó, D.; Szekrényes, D. P.; Pothorszky, S.; Nagy, N.; Deák, A. SERS Activity of Reporter-Particle-Loaded Single Plasmonic Nanovoids. J. Phys. Chem. C 2018, 122 (41), 23683–23690. (IF = 4.189)
Table of links
KFKI: https://www.kfki.hu/
Centre for Energy Research: https://www.energia.mta.hu
Institute of Technical Physics and Materials Science: https://www.mfa.kfki.hu/
Chemical Nanostructures Laboratory: https://www.energia.mta.hu/~deak/
Localised surface plasmon resonance: https://en.wikipedia.org/wiki/Localized_surface_plasmon
Resonance: https://en.wikipedia.org/wiki/Resonance
Surface enhanced Raman spectroscopy: https://en.wikipedia.org/wiki/Surface-enhanced_Raman_spectroscopy
cysteamine: https://en.wikipedia.org/wiki/Cysteamine
3-mercaptopropionic acid: https://en.wikipedia.org/wiki/3-Mercaptopropionic_acid
Hexadecyl(trimethyl)ammonium bromide: https://en.wikipedia.org/wiki/Cetrimonium_bromide
UV-Vis spectroscopy: https://en.wikipedia.org/wiki/Ultraviolet%E2%80%93visible_spectroscopy
Electrophoretic mobility: https://en.wikipedia.org/wiki/Electrophoresis
Scanning electron microscopy: https://en.wikipedia.org/wiki/Scanning_electron_microscope
Single particle spectroscopy: https://www.nature.com/articles/s41586-020-2048-8
Atomic force microscopy: https://en.wikipedia.org/wiki/Atomic_force_microscopy
Dark-field microscopy: https://www.olympus-lifescience.com/en/microscope-resource/primer/techniques/darkfield/
Inverted optical microscopy: https://en.wikipedia.org/wiki/Inverted_microscope
(11-mercaptoundecyl)-N,N,N-trimethylammonium bromide:
https://pubchem.ncbi.nlm.nih.gov/compound/71310936
List of references
[1] Petryayeva, E.; Krull, U. J. Localized Surface Plasmon Resonance: Nanostructures, Bioassays and Biosensing—A Review. Analytica Chimica Acta 2011, 706 (1), 8–24.
[2] Taylor, R. W.; Esteban, R.; Mahajan, S.; Coulston, R.; Scherman, O. A.; Aizpurua, J.; Baumberg, J. J. Simple Composite Dipole Model for the Optical Modes of Strongly-Coupled Plasmonic Nanoparticle Aggregates. J. Phys. Chem. C 2012, 116 (47), 25044–25051.
[3] Häkkinen, H. The Gold–Sulfur Interface at the Nanoscale. Nature Chemistry 2012, 4 (6), 443–455.
[4] Zámbó, D.; Pothorszky, Sz.; Brougham, D. F.; Deák, A. Aggregation Kinetics and Cluster Structure of Amino-PEG Covered Gold Nanoparticles. RSC Adv. 2016, 6 (32), 27151–27157.
[5] Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C. B. Using Binary Surfactant Mixtures To Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Letters 2013, 13 (2), 765–771.
[6] Pothorszky, Sz.; Zámbó, D.; Deák, T.; Deák, A. Assembling Patchy Nanorods with Spheres: Limitations Imposed by Colloidal Interactions. Nanoscale 2016, 8 (6), 3523–3529.
[7] Zhang, W.; Li, Q.; Qiu, M. A Plasmon Ruler Based on Nanoscale Photothermal Effect. Opt. Express 2013, 21 (1), 172.
