|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Organic Chemistry and Technology
Supervisor: Dr. Csontos István
Continuous formulation of electrospun samples
Introducing the research area
The number of effective drug candidates is increasing year by year thanks to innovative molecule searching and testing methods. However, the majority of the promising active pharmaceutical ingredients are poorly water-soluble, thus their dissolution and bioavailability are low, which hinders authorization processes and marketing. An excellent way of improving dissolution is electrospinning, a fast, gentle, and continuous technology for preparing nano and micro size fibers with appropriate drug release. Despite its advantageous properties, the method has only been applied in pharmaceutical research and development as yet. The main reason for this is that the effective and safe scaled-up production of the fibrous materials, the downstream processing and formulation of the final dosage forms have not been fully elaborated. Therefore, the research aimed to prepare electrospun material-loaded tablets continuously by integrating multiple technological steps. Further goal was to continuously monitor the manufacturing with non-destructive analytical tools to ensure constant product quality.
Brief introduction of the research place
The FirePharma research group at the Department of Organic Chemistry and Technology of the Faculty of Chemical Technology and Biotechnology deals with innovative topics of international interest in the field of the pharmaceutical industry, of which several publications have been published in high impact factor journals. Our group is in contact with and participates in joint projects with domestic and foreign pharmaceutical companies and academic research groups.
History and context of the research
Nowadays, a great portion of the potential drug candidates has poor water solubility, thus the dissolution is low, which usually leads to low bioavailability. To tackle the solubility problems, several methods have been developed over the years, including the preparation and application of amorphous solid dispersions[1]. The apparent solubility and thus the bioavailability can be increased via the formation of the amorphous structure, while the chemical structure and the effect of the active pharmaceutical ingredient does not change. The success of the use of amorphous solid dispersions is indicated by the fact that by 2017, 24 amorphous solid dispersion-loaded medicines have been approved by the U.S. Food and Drug Administration [2].
Over the years, several technological solutions have been developed for the preparation of amorphous solid dispersions, among which solution-based electrospinning is a highly effective method. However, the pharmaceutical application of the technology requires scaling-up, the downstream processing of the nano and micro-sized fibers and the preparation of the most common dosage form, the tablets [3,4]. Furthermore, it would be desirable to form the final dosage form from the electrospun amorphous solid dispersions through continuous technological steps, thereby reducing manufacturing times, costs, and space requirements of the manufacturing line. The pharmaceutical agencies are also increasingly supporting continuous technologies and the associated quick, non-destructive analytical methods. Various guidelines encourage pharmaceutical companies to use continuous technologies to make the processes more controllable, safer, and efficient [5].
The joint application of amorphous solid dispersions and continuous technologies allows the efficient, environmentally friendly, and safe production of constant quality tablets containing poorly water-soluble active pharmaceutical ingredients. As a result, a lot of new, effective pharmaceutical formulations may be available to patients, while these medicines are produced using state-of-the-art methods.
The research goals, open questions
Although the pharmaceutical application opportunities of electrospinning are widely researched, only a few examples can be found in the literature for the manufacture of tablets containing fibers and continuous downstream processing of fibers has not been realized as yet. For this reason, the research aimed to enhance the dissolution of two poorly water-soluble active pharmaceutical ingredients, namely the spironolactone and the itraconazole, using electrospinning, and then to produce tablets from the fibers by continuous processing steps. A further goal of the work was to develop non-destructive, in-line applicable analytical methods for the detection of crystalline traces and for measuring the drug content, thus ensuring consistent product quality during the continuous production of tablets containing fibers.
Methods
Electrospinning
For fiber production, the high-speed electrospinning machines developed in our department were used. The spironolactone-loaded samples were prepared using horizontally arranged equipment with a round-shaped stainless-steel spinneret. The solution flowed through equidistant orifices in the sidewall of the spinneret, while the spinneret made a rotating motion. The spinneret was connected to high voltage and a grounded collector was located opposite the spinneret. Due to the potential difference and the centrifugal forces, the outgoing liquid jet was elongated and thinned, the solvent instantly evaporated, and nano- and micro-diameter fibers were formed on the grounded collector. The vertically arranged equipment used for preparing the itraconazole-loaded samples was connected to a cyclone (Figure 1). When installed in the proper environment, this equipment can meet GMP (Good Manufacturing Practice) requirements, a key consideration in pharmaceutical industry. In both cases, the dosing of the solutions was performed with a peristaltic pump.
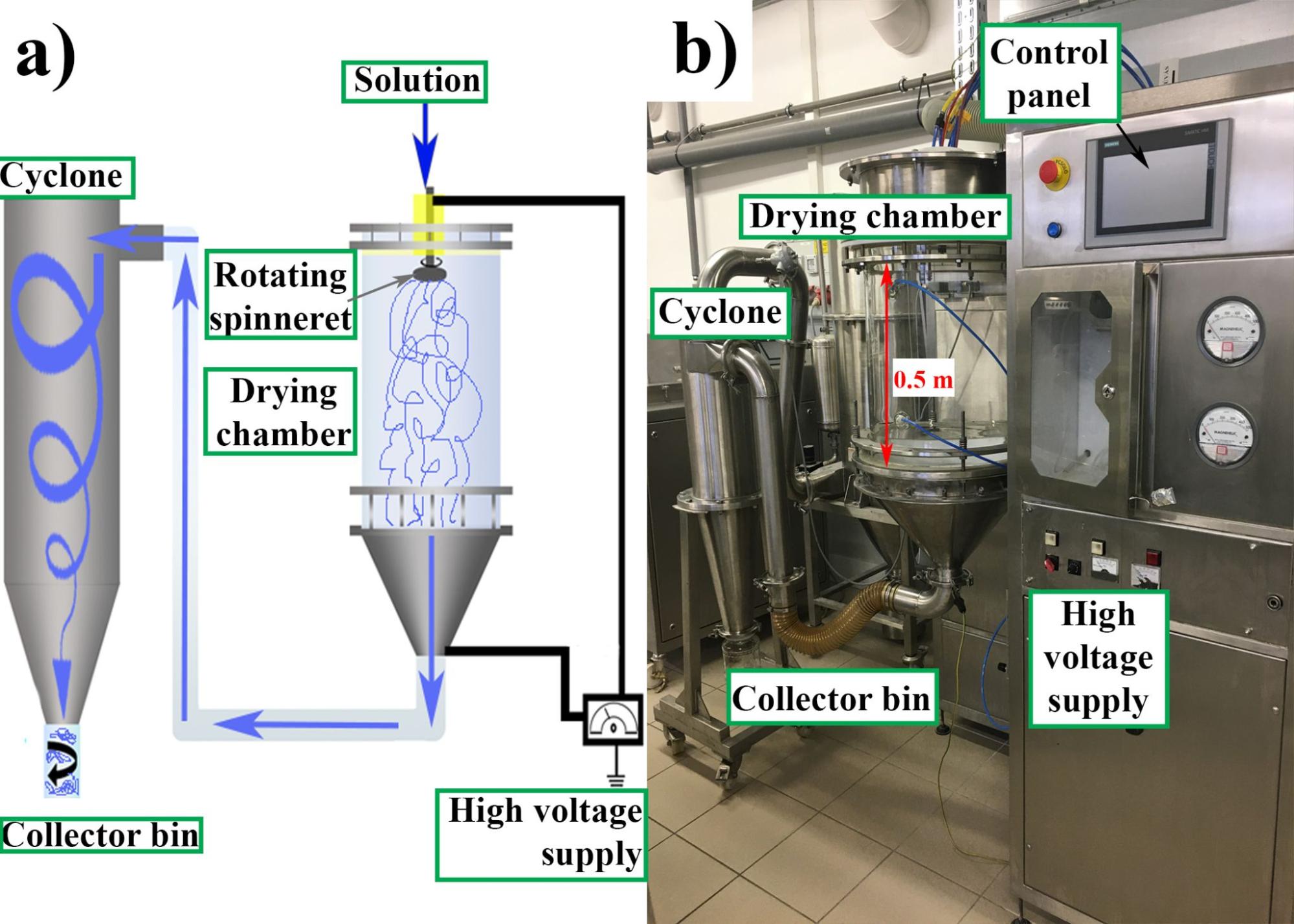
Figure 1 High-speed electrospinning apparatus coupled with a cyclone (schematic drawing (a) and photo (b))
Formulation of fibers
The spironolactone-loaded fibers were collected with a piece of equipment designed in our department that consisted of three rotating rolls to help move the collection material continuously, while a medical blade at the bottom of the device performed continuous removal of the fibers. The cyclone shown in Figure 1 was used for the continuous collection of itraconazole-loaded fibers. Spironolactone-loaded fibers were milled using a sieve with a hole size of 0.8 mm, while the itraconazole-loaded fibers were ground with an oscillating milling device using a sieve with 2.0 mm holes. For the feeding experiments, a twin-screw gravimetric feeder and a vibratory feeder were used. The continuous blending was performed with a twin-screw extruder from which a conveyor belt transported the powder mixture to the tableting machine. Electrospun materials-containing tablets were produced using an eccentric tableting machine in both cases.
Analytical methods
The fibers were analyzed by scanning electron microscopy, differential scanning calorimetry, X-ray powder diffraction, thermogravimetric analysis, and a dissolution tester coupled with UV–Vis spectrophotometry. The particle size distribution of the milled fibers was determined by laser diffraction measurement. Furthermore, the bulk and tapped density of the blends, and the brittleness, attrition, thickness, and moisture content of the tablets were also examined.
In addition, near-infrared (NIR) and Raman spectroscopy based non-destructive analytical methods were developed for potential use in in-line continuous manufacturing. Based on the spectroscopic data, calibration models were built with MATLAB software to measure crystalline traces and the drug content.
Results
Using high-speed electrospinning resulted in more than thirty-fold productivity growth in the case of spironolactone-loaded fibers. Continuous collection of the fibers was successfully achieved with the device designed in our department and the fibers were easy to grind. The blend of the spironolactone-loaded fibers and large particle size microcrystalline cellulose proved to be continuously feedable using a twin-screw gravimetric feeder at a feed rate of 385 g/h. The blending of the powder mixture with further excipients enabled the preparation of tablets containing drug-loaded fibers. The dissolution of the tablets was similar to that of the pure electrospun samples. I found that the crystalline content resulted in lower dissolution, thus NIR and Raman spectroscopic methods were developed to detect the crystalline traces. The best calibration results were obtained from the Raman spectroscopic investigation of the blends, which confirmed that non-destructive, in-line analytical tools are suitable for product quality monitoring during continuous manufacturing. The experimental results confirmed the feasibility of continuous downstream processing of drug-loaded electrospun fibers; however, it is essential to develop rapid analytical methods linked with the processes to ensure proper quality end products (Figure 2).
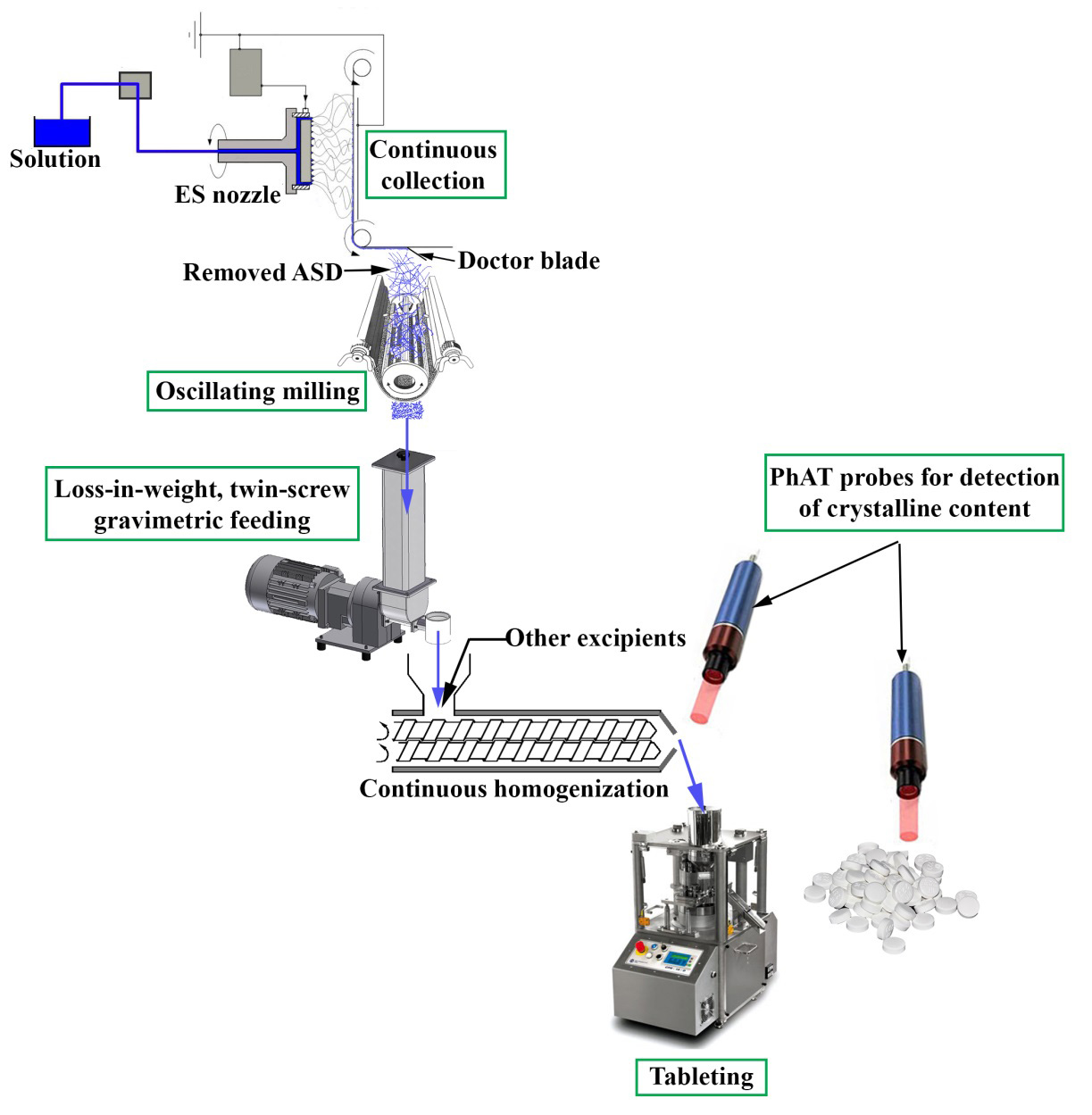
Figure 2. Schematic design of a continuous line showing the process steps from electrospinning to directly compressed tablets. (Green rectangles mark the areas involved in this work).
The next step in the research was to achieve continuous blending and tableting, for which itraconazole-loaded fibers were prepared (Figure 3).
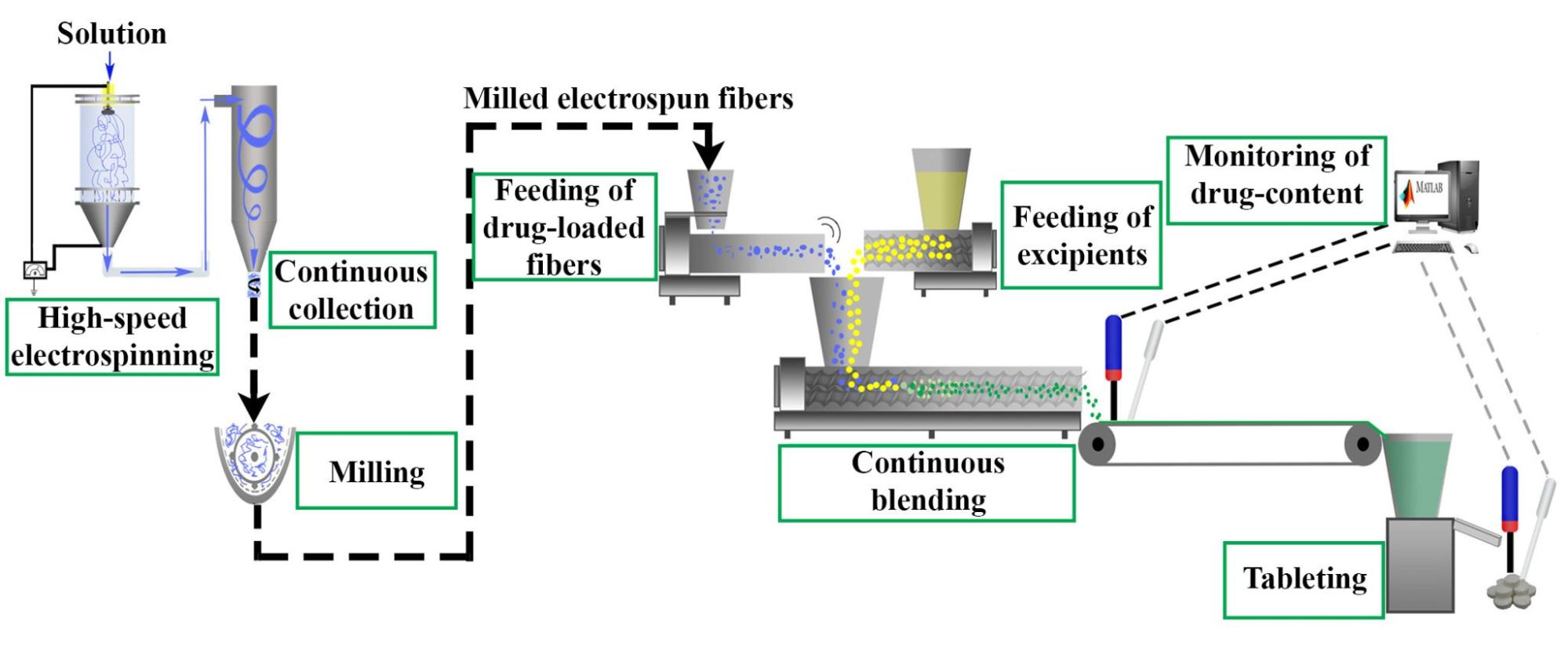
Figure 3. Schematic drawing of an integrated system developed for the continuous production of tablets containing itraconazole-loaded fibers.
The continuous collection of the fibers was performed with a cyclone. During the production, the fibers made a continuous rotational movement in the collector bin placed at the bottom of the cyclone (Figure 4).

Figure 4. The fibers performed a continuous rotational motion in the collector bin located at the bottom of the cyclone.
Although the fibers were continuously ground in the collector bin as a result of the rotational motion, an additional milling step was required to enhance flowability and handling. For this purpose, oscillating milling, which can be incorporated into continuous manufacturing lines, proved to be suitable. In the next step, the fibers without excipients were successfully fed into a twin-screw blender using a vibratory feeder, while the mixture of the excipients was dosed with a twin-screw gravimetric feeder (Figure 5).
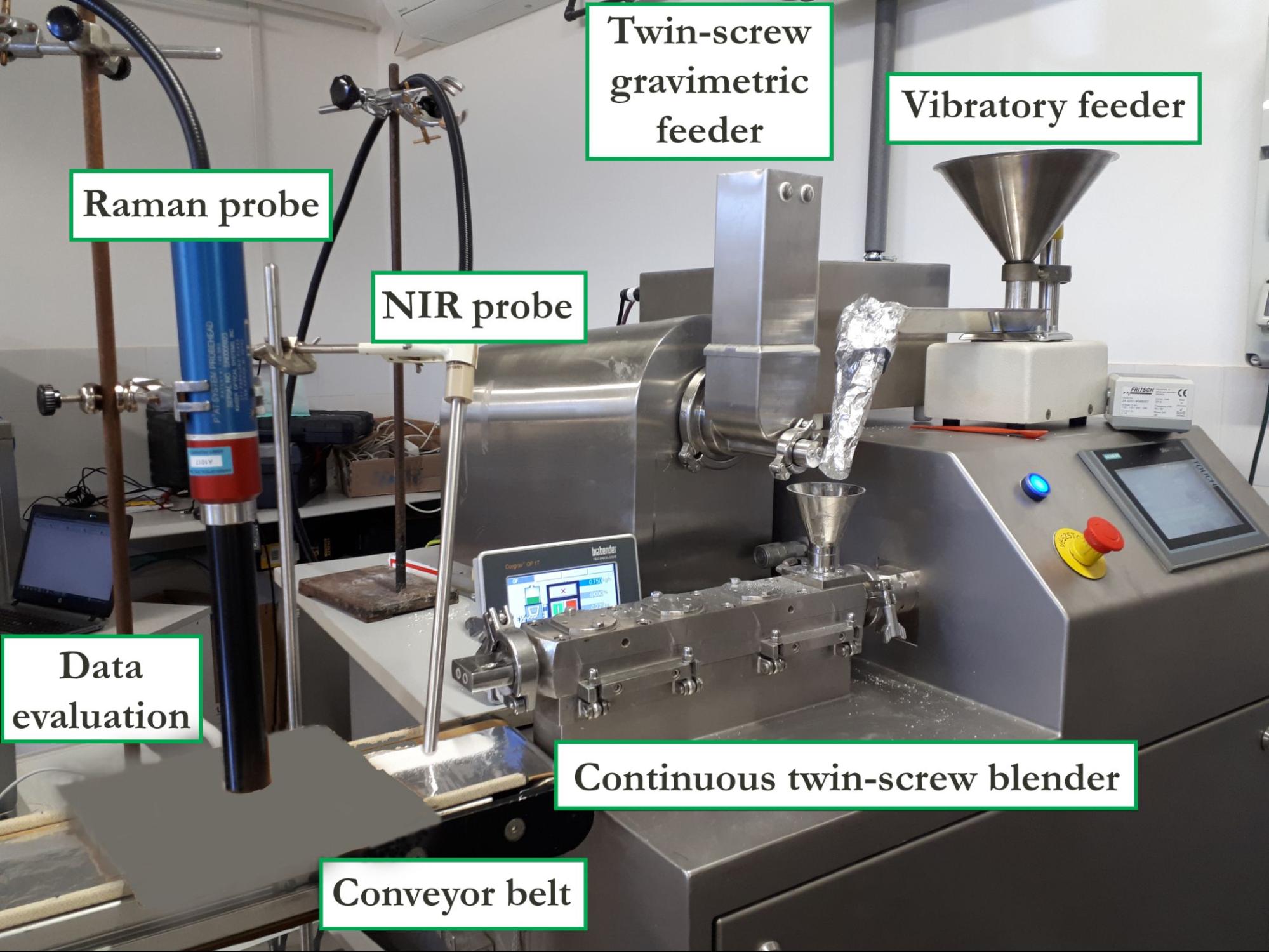
Figure 5. Photo of the continuous blending experimental setup.
The fibrous material content of the blend and thus the drug content was continuously measured with NIR and Raman spectroscopy (Figure 6). Finally, a conveyor belt transported the blend to the tableting machine. At the end of the process, the drug content of the prepared tablets could also be successfully determined with NIR and Raman spectroscopy. Furthermore, the UV–Vis spectrophotometry used as a reference method confirmed the results of the two non-destructive analytical methods.
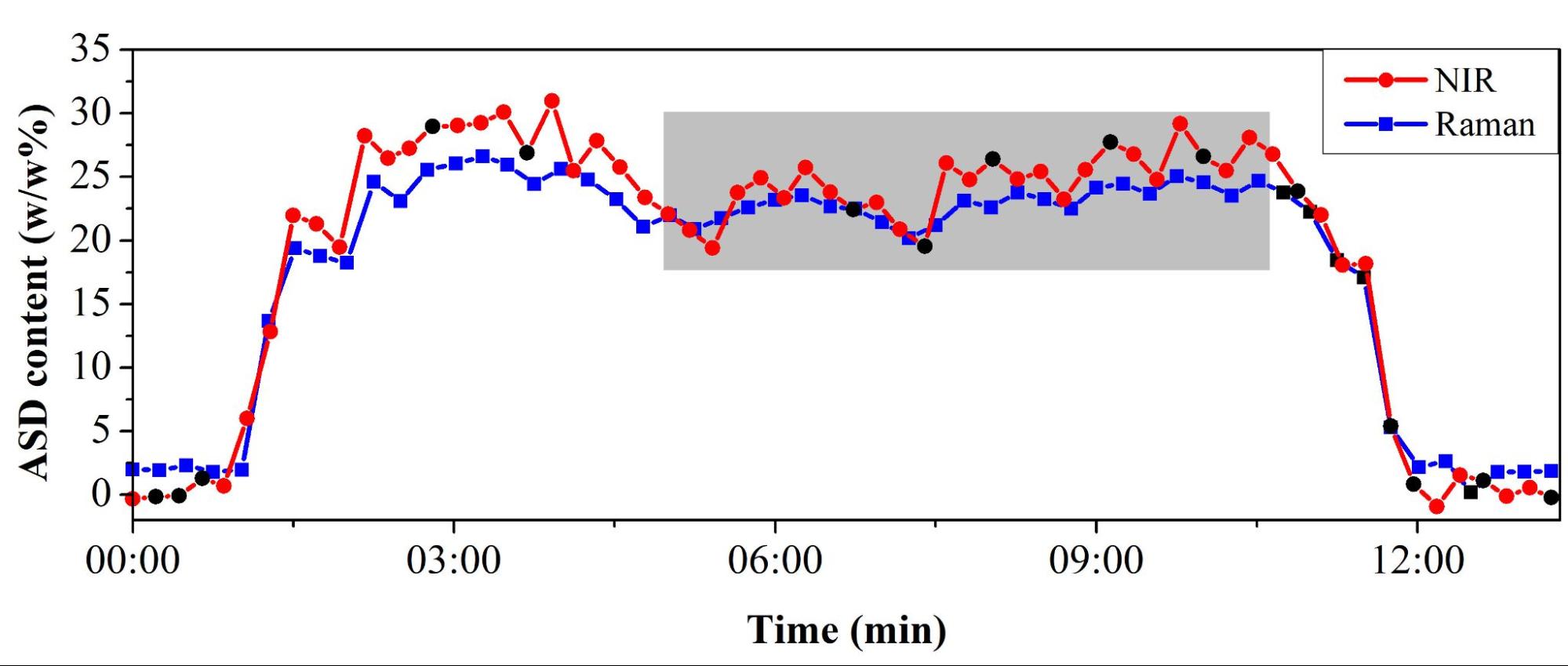
Figure 6. Monitoring of continuous blending by NIR and Raman spectroscopy. (Gray background indicates the period when the feeding set up and the system reached a steady state.)
Expected impact and further research
The results of the research demonstrated the feasibility of continuous downstream processing of electrospun fibers, which allows enhancing the dissolution of poorly water-soluble drugs and their effective, fast, and safe production in tablet form. A significant part of the work was carried out in collaboration with the scientists of Richter Gedeon Plc. in the frame of FIEK_16-1-2016-0007 project. Furthermore, certain parts of the work were conducted in collaboration with the scientists of Janssen Pharmaceutica (Pharmaceutical Companies of Johnson & Johnson). All of these collaborations suggest a strong industrial interest in the topic, and that the results can be utilized in the near future.
As a continuation of the research, I investigate the possibilities of the continuous granulation of fibers and the factors influencing the stability of amorphous solid dispersions to make the production of tablets and capsules easier and more efficient.
Publications, references, links
List of corresponding own publications (IF: impact factor, C: citations)
[I] E. Szabó, B. Démuth, B. Nagy, K. Molnár, A. Farkas, B. Szabó, A. Balogh, E. Hirsch, G. Marosi, Z.K. Nagy, Scaled-up preparation of drug-loaded electrospun polymer fibres and investigation of their continuous processing to tablet form, Express Polymer Letters, 12(5) (2018), 436–451
IF: 2.875 C: 27
[II] E. Szabó, B. Démuth, D.L. Galata, P. Vass, E. Hirsch, I. Csontos, G. Marosi, Z.K. Nagy, Continuous formulation approaches of amorphous solid dispersions: Significance of powder flow properties and feeding performance, Pharmaceutics, 11(12) (2019), 654
IF: 4.421 C: 10
[III] E. Szabó, P. Záhonyi, D. Brecska, D.L. Galata, L.A. Mészáros, L. Madarász, K. Csorba, P. Vass, E. Hirsch, J. Szafraniec-Szczęsny, I. Csontos, A. Farkas, G. Van den Mooter, Z.K. Nagy, G. Marosi, Comparison of amorphous solid dispersions of spironolactone prepared by spray drying and electrospinning: The influence of the preparation method on the dissolution properties, Molecular Pharmaceutics, 18(1) (2021), 317–327
IF: 4.939 C: 0
[IV] E. Szabó, P. Záhonyi, M. Gyürkés, B. Nagy, D.L. Galata, L. Madarász, E. Hirsch, A. Farkas, S.K. Andersen, T. Vígh, G. Verreck, I. Csontos, G. Marosi, Z.K. Nagy, Continuous downstream processing of milled electrospun fibers to tablets monitored by near infrared and Raman spectroscopy, European Journal of Pharmaceutical Sciences, 164 (2021), 105907
IF: 4.384 C: 0
[V] P. Vass, E. Szabó, A. Domokos, E. Hirsch, D. Galata, B. Farkas, B. Démuth, S.K. Andersen, T. Vigh, G. Verreck, G. Marosi, Z.K. Nagy, Scale‐up of electrospinning technology: Applications in the pharmaceutical industry, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 12(4) (2020), e1611
IF: 9.182, C: 53
[VI] B. Démuth, D.L. Galata, E. Szabó, B. Nagy, A. Farkas, A. Balogh, E. Hirsch, H. Pataki, Z. Rapi, L. Bezúr, T. Vigh, G. Verreck, Z. Szalay, Á. Demeter, G. Marosi, Z.K. Nagy, Investigation of deteriorated dissolution of amorphous itraconazole: Description of incompatibility with magnesium stearate and possible solutions, Molecular Pharmaceutics, 498 (2017), 234–244
IF: 4.556 C: 14
[VII] B. Démuth, D.L. Galata, A. Balogh, E. Szabó, B. Nagy, A. Farkas, E. Hirsch, H. Pataki, T. Vígh, J. Mensch, G. Verreck, Z.K. Nagy, G. Marosi, Application of hydroxypropyl methylcellulose as a protective agent against magnesium stearate induced crystallization of amorphous itraconazole, European Journal of Pharmaceutical Sciences, 121 (2018), 301–308
IF: 3.532 C: 8
[VIII] P. Vass, E. Hirsch, R. Kóczián, B. Démuth, A. Farkas, C. Fehér, E. Szabó, Á. Németh, S.K. Andersen, T. Vígh, G. Verreck, I. Csontos, G. Marosi, Z.K. Nagy, Scaled-up production and tableting of grindable electrospun fibers containing a protein-type drug, Pharmaceutics, 11(7) (2019), 329
IF: 4.421 C: 13
[IX] P. Vass, Z.K. Nagy, R. Kóczián, C. Fehér, B. Démuth, E. Szabó, S.K. Andersen, T. Vígh, G. Verreck, I. Csontos, G. Marosi, E. Hirsch, Continuous drying of a protein-type drug using scaled-up fiber formation with HP-β-CD matrix resulting in a directly compressible powder for tableting, European Journal of Pharmaceutical Sciences, 141 (2020), 105089
IF: 4.384 C: 12
[X] G. Fülöp, A. Domokos, D.L. Galata, E. Szabó, M. Gyürkés, B. Szabó, A. Farkas, L. Madarász, B. Démuth, T. Lendér, T. Nagy, D. Kovács-Kiss, F. Van der Gucht, G. Marosi, Z.K. Nagy, Integrated twin-screw wet granulation, continuous vibrational fluid drying and milling: A fully continuous powder to granule line, International Journal of Pharmaceutics, 594 (2021), 120126
IF: 5.875 C: 3
Table of links
Department of Organic Chemistry and Technology
List of references
[1] G. Van den Mooter, The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate, Drug Discov. Today, 9(2) (2012), e79-e85
[2] S.V.Jermain, C. Brough, R.O. Williams III, Amorphous solid dispersions, and nanocrystal technologies for poorly water-soluble drug delivery – An update, Int. J. Pharm., 535(1–2) (2018), 379–392
[3] Z.K. Nagy, A. Balogh, B. Démuth, H. Pataki, T. Vígh, B. Szabó, K. Molnár, B.T. Schmidt, P. Horák, G. Marosi, G. Verreck, I. Van Assche, M.E. Brewster, High speed electrospinning for scaled-up production of amorphous solid dispersion of itraconazole, Int. J. Pharm., 480(1–2) (2015), 137–142
[4] B. Démuth, Z.K. Nagy, A. Balogh, T. Vígh, G. Marosi, G. Verreck, I. Van Assche, M.E. Brewster, Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations, Int. J. Pharm., 486(1–2) (2015), 268–286
[5] S.L. Lee, T.F. O’Connor, X. Yang, C.N. Cruz, S. Chatterjee, R.D. Madurawe, C.M. V Moore, L.X. Yu, J. Woodcock, Modernizing pharmaceutical manufacturing: from batch to continuous production, J. Pharm. Innov. 10 (2015) 191–199.
