|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
ELRN Research Centre for Natural Sciences, VBK Department of Inorganic and Analytical Chemistry
Supervisor: Dr. Turiák Lilla
Deciphering new molecular markers for prostate cancer diagnostics
Introducing the research area
Proteoglycans are macromolecules composed of a core protein and sulfated linear polysaccharides attached to it. They are involved in the structural maintenance of the extracellular matrix and the coordination of intercellular communication. Thus, they play a key role in the development of cancer and metastasis formation. In the course of my research project, I investigate whether biochemical changes can be detected in this molecular family that may facilitate a more accurate risk assessment of prostate cancer, thereby helping therapeutic decision-making. I use self-developed tissue sample preparation and HPLC-MS measurement methods for my experiments, thus allowing the examination of small amounts of tissue biopsies.
Brief introduction of the research place
I conduct my research in the MS Proteomics Research Group of the Research Centre for Natural Sciences under the supervision of Lilla Turiák, Ph.D. The focus of our group is on the structural and quantitative investigation of proteins and their post-translation modifications in the course of several diseases. Collaborations with national and international pathological centers are formed during our research. The investigations are carried out on cutting-edge mass spectrometry platforms.
History and context of the research
Prostate cancer accounts for 14.1% of tumorous lesions in men, making it one of the most common cancer types [H1]. In terms of mortality, it is considered to be moderate, largely due to routine screening for prostate-specific antigen (PSA) blood levels in the population over the age of 50 years. Current risk assessment methods of prostate cancer patients are based on PSA level, the clinical tumor stage and Gleason score describing cell differentiation. However, these methods lead to unnecessary interventions (primarily radical prostatectomy) in many cases, the unpleasant side effects of which could be avoided by choosing different therapeutic routes. However, the identification of new markers is needed to create more sophisticated risk assessment groups.
The family of proteoglycans (PGs) is a promising candidate for this, as they have previously been associated with the development of several tumor lesions [H2].
Proteoglycans are macromolecules in which sulfated linear polysaccharide chains (glycosaminoglycans, GAGs) composed of repeating disaccharide units are attached to distinguished core proteins. PGs are involved in building the extracellular matrix and coordinating intercellular signaling transduction processes. Thus, they play a significant role in developing tumors and metastasis formation. The number of attached GAG chains, their chain length, and the degree and position of sulfation contribute significantly to these functions.
Four different GAG classes are distinguished based on the type of the repeating disaccharide units, as follows: i) hyaluronan, ii) keratan sulfate, iii) chondroitin sulfate/dermatan sulfate (CS/DS), iv) heparin/heparan sulfate (Hep/HS). I perform my research on the CS and HS classes.
Techniques involving mass spectrometry (especially capillary ultra-high performance liquid chromatography-tandem mass spectrometry, nano HPLC-MS/MS) are excellent for the qualitative and quantitative analysis of samples originating from complex biological matrices. Components are separated based on specific physicochemical parameters (e.g. hydrophobicity, ionization state), so they arrive in a time-resolved manner in the hyphenated mass spectrometer. The MS can be used to determine the mass-to-charge ratio (m/z) and the intensity of the molecules, allowing parallel qualitative and quantitative analysis. However, the investigation of GAGs from small tissue samples requires the development and application of specific methods for both sample preparation and measurement side.
The research goals, open questions
The first goal of my research was to develop sample preparation and nanoUHPLC-MS/MS methods for the investigation of tissue glycosaminoglycans. The optimization of the digestion and extraction processes proved to be necessary along with the development of new solid-phase extraction methods and HPLC-MS methods, which are suitable for the reliable analysis of the GAG composition of small-size tissue sections.
After developing the methods, my goal was to investigate tissue biopsies from patients with varying severity of prostate cancer (PCa) and benign prostatic hyperplasia (BPH). These studies had a dual purpose: (i) to identify molecular processes that are in line with current risk classifications and allow the identification of potential therapeutic targets, and (ii) to identify (independent) markers for the development of more detailed risk assessment methods to reduce overtreatment. Thus, in evaluating the measurement data, my goal is to construct multivariate models to explore new glycosaminoglycan-based markers for the risk assessment of prostate cancer lesions.
Methods
Sample collection
Samples and patient data were collected at the Urology Clinic of the Essen Hospital (Germany) under the guidance of Dr. Tibor Szarvas. The cohort was balanced on case numbers, clinical parameters, age, survival time, and tumor cell ratio. I investigated formalin-fixed, paraffin-embedded (FFPE) tissue samples from patients with benign prostatic hyperplasia (n = 14), low (n = 20), moderate (n = 20), and high (n = 17) risk prostate cancer patients. Investigation of FFPE samples allows the examination of diseases with a long survival time due to the durability of the tissues.
Tissue preparation
As a first step, I removed the paraffin from the tissues using a xylene-ethanol washing process widely used in the literature. Then, the so-called antigen retrieval was performed by boiling the tissue slides in an aqueous solution of sodium citrate for 30 min. This allowed the break-up of the crosslinks created by formalin between tissue macromolecules so that the enzymes have access to the given molecules during enzymatic degradation (digestion).
Enzymatic digestion of GAG chains
Digestion was performed with chondroitinase-ABC enzyme solution (pH=7.6) for CS chains and Heparin lyase I-II-III (pH=7.6) for HS chains. The addition of enzyme solutions to the tissue surface was performed cyclically (5 equal timeframes during 24h and 48h digestion, respectively), thus ensuring complete conversion of tissue GAG chains [S1, S2]. The disaccharides formed during the digestion (Figure 1) were then removed from the tissue surface by five-cycle repeated pipetting of 1% ammonia solution, and then the solvents were evaporated. For evaporation, I used a self-developed method to ensure the lowest possible loss without distortion in the relative ratios of sulfated disaccharides [S8].

Figure 1. Enzymatic digestion of CS and HS chains and the resulting sulfated disaccharide repeating units. The so-called Lawrence code is shown beside the respective disaccharides.
Solid-phase extraction purification
The next step was to purify the digestion mixture from buffer components and enzymes using solid-phase extraction (SPE). During SPE, the sample is applied to a solid stationary phase in the form of a solution. The target compounds are bound to the stationary phase, while other compounds can be removed during the washing step. Subsequently, GAG disaccharides were eluted from the stationary phase. For purification, we have developed a method that allows the undistorted purification of GAG disaccharides by a combination of an environmentally friendly self-packed cotton stationary phase and a commercially available graphite-based stationary phase (publication in preparation).
nanoUHPLC-MS/MS measurements
After further evaporation [S8], samples were reconstituted in the injection solvent and nanoUHPLC-MS/MS measurements were performed. Using a self-packed capillary column based on hydrophilic interaction chromatography and weak anion exchange (HILIC-WAX), I developed an innovative salt gradient method for the analysis of heparan sulfate and chondroitin sulfate disaccharides [S4, S5]. Using this method, GAG disaccharides can be measured at detection limits up to ten times lower than before, thus allowing the investigation of small-size tissues. A summary of the workflow is shown in Figure 2.
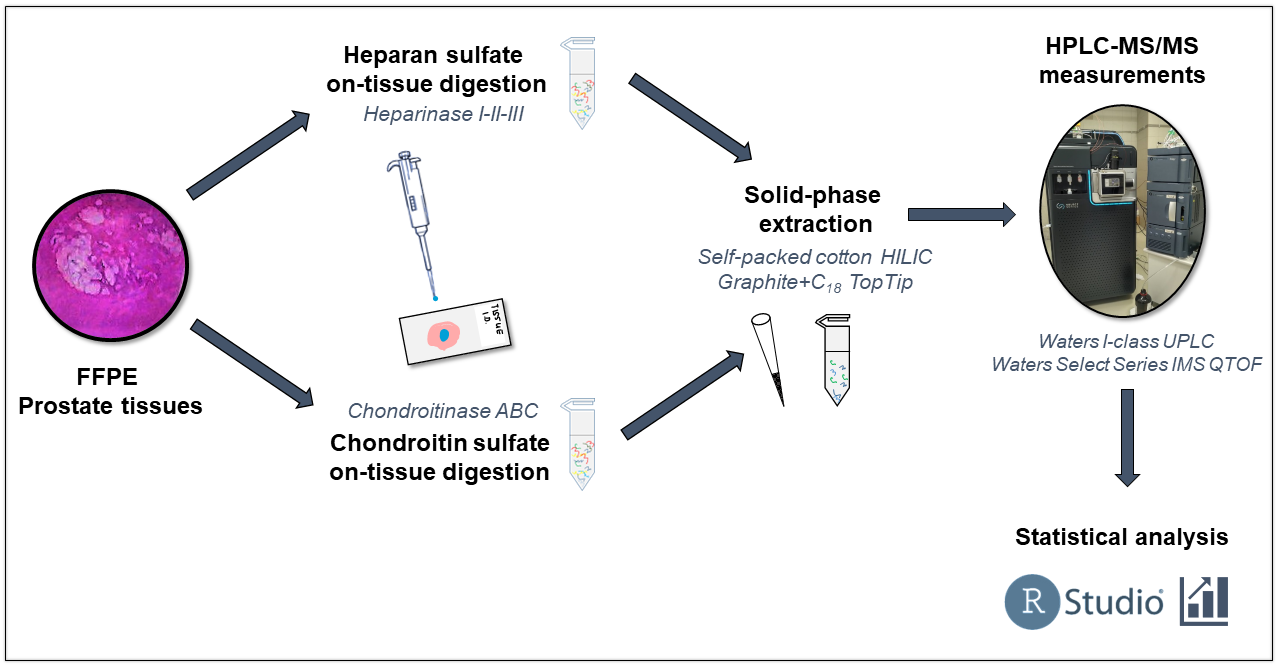
Figure 2. The brief workflow for the sample preparation, HPLC-MS measurement, and data evaluation of prostate cancer samples.
Data analysis
Analysis of the results was performed by Kaplan-Meier (KM) analysis following general statistical group comparisons. GAG motifs with significant differences in survival were then subjected to multivariate analysis to determine their independence from clinical parameters currently used for risk assessment.
Results
Correlations with current risk assessment methods
I identified a 1.6–2.0-fold significant (p <0.05) increase in the total amount of CS chains in PCa tissues compared to BPH. No statistically significant differences were observed between the individual risk groups, however, there was a small increase in mean and median with the progression of PCa (Figure 3). There was also a small increase (1.3–1.4-fold) in the total amount of HS chains in PCa tissues compared to BPH (Figure 3).
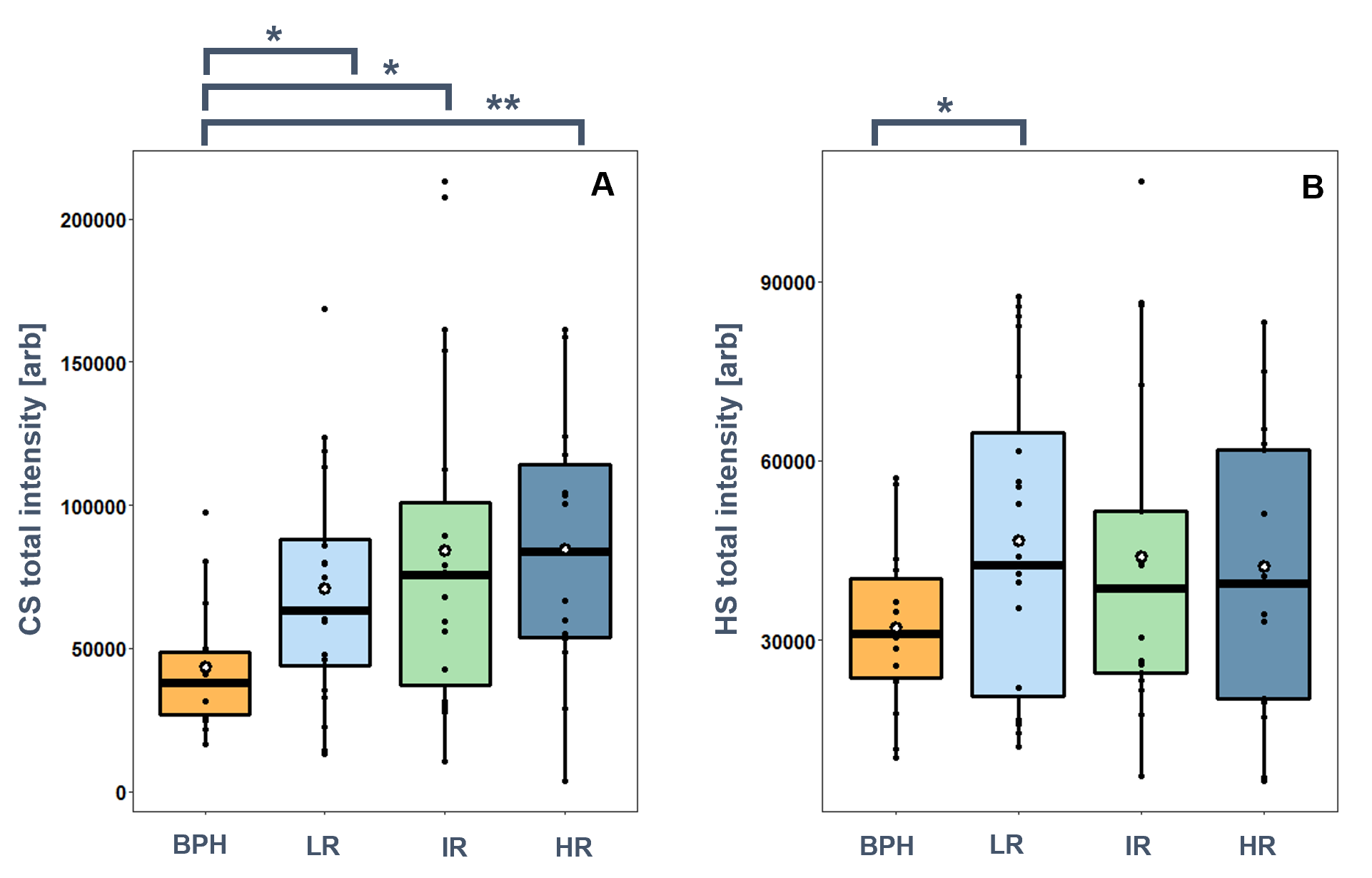
Figure 3. Total GAG content in BPH, and low-risk (LR), intermediate-risk (IR), and high-risk (HR) prostate cancer. A: chondroitin sulfate, B: heparan sulfate. (*:p<0.05, **:p<0.01).
Comparing the relative amounts of the GAG disaccharides, I found that the most significant differences were found in the relative intensities of the monosulfated CS disaccharides (Figure 4/A). The relative intensity of D0a4 disaccharide is significantly lower in PCa than in BPH, and a further decreasing trend is observed with the progression of PCa. D0a6 disaccharide showed an opposite trend: a small increase compared to BPH was observed in the low-risk group, but a large increase was observed with PCa progression (Figure 4/B).
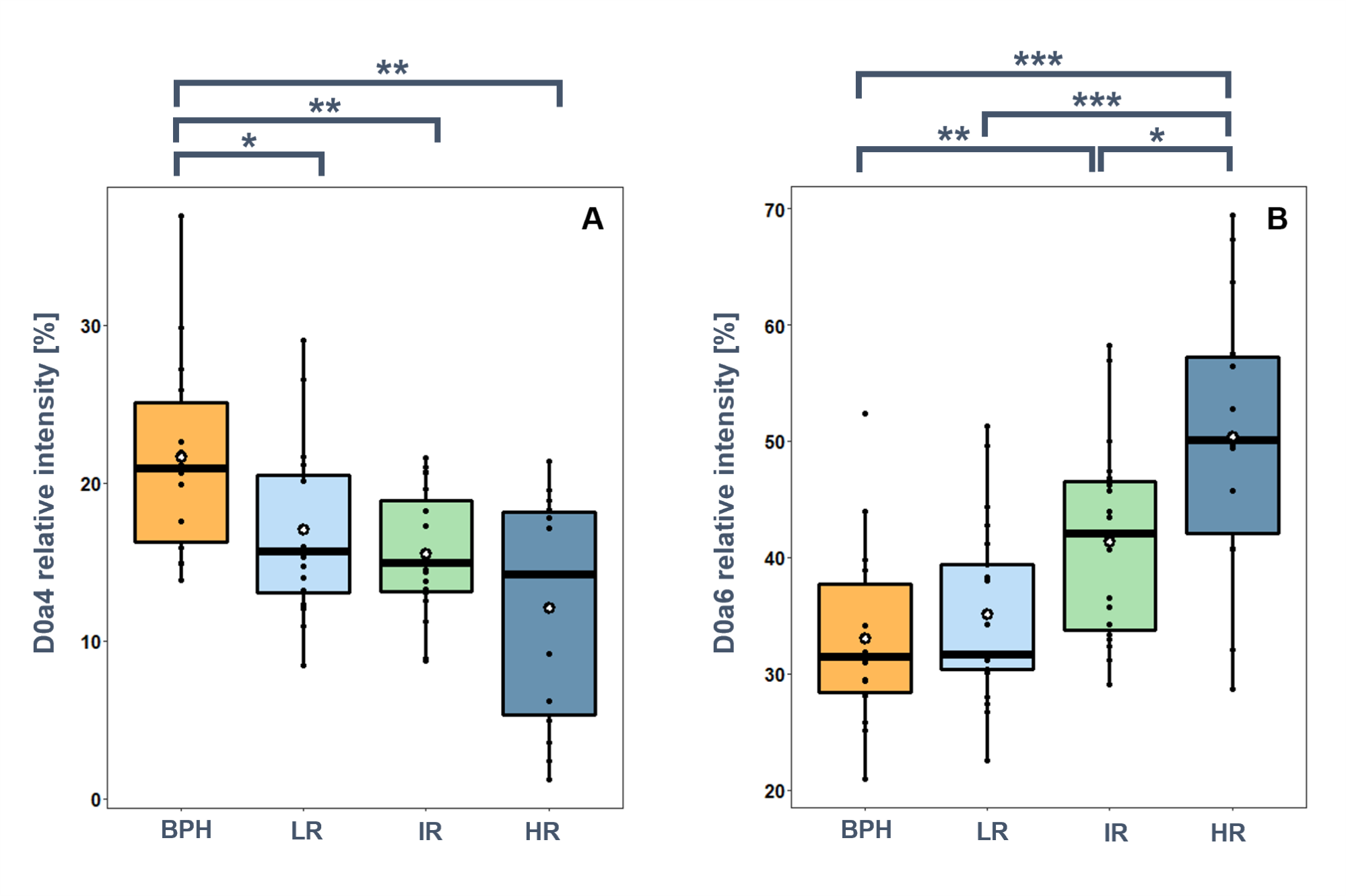
Figure 4.
Relative intensities of selected CS disaccharides in BPH, and low-risk (LR),
intermediate-risk (IR), and high-risk (HR) prostate cancer. A: D0a4, B: D0a6.
(*:p<0.05, **:p<0.01, ***:p<0.001).
Our results support that an increase in the amount of CS and 6-O-sulfation may be associated with tissue rearrangement and tumor progression [H3]. Chondroitin-6-sulfate (C-6-S) inhibits the release of pro-inflammatory molecules from macrophages, inhibits a significant proportion of inflammatory mediators, and inhibits NF-κB activation so that increasing amounts of C-6-S maintain M2 polarization of cells, thus promoting tumor survival [H4].
Smaller and less significant changes were detected for HS chains. The most significant of these is the decrease in the average sulfation and the predominance of N-sulfation over O-sulfation in correlation with the increasing risk of prostate cancer.
Survival estimation - Kaplan-Meier Analysis
To investigate the relationship between the survival probability of prostate cancer patients and the levels of GAG motifs, disease-specific Kaplan-Meier (KM) survival curves were prepared. The KM curves show the time at which mortality associated with prostate cancer occurred after the time of sampling. Patients who survived or suffered non-prostate cancer-related death were considered censored data.
I found that significantly different survival groups (logrank P <0.05) can be formed based on the relative intensity of D0a10 disulfated CS disaccharide and the total HS content, and the relative intensity of D2S0/D0S6 and D2S6 HS disaccharides (Figure 5). Concerning GAG motifs showing significant differences in survival, no correlation was observed with currently used PCa risk groups. There is a slight downward trend in all cases with an increase in the risk group, which is consistent with the data from the KM analysis. In most cases, the lower amount of a given GAG motif is associated with shorter survival.
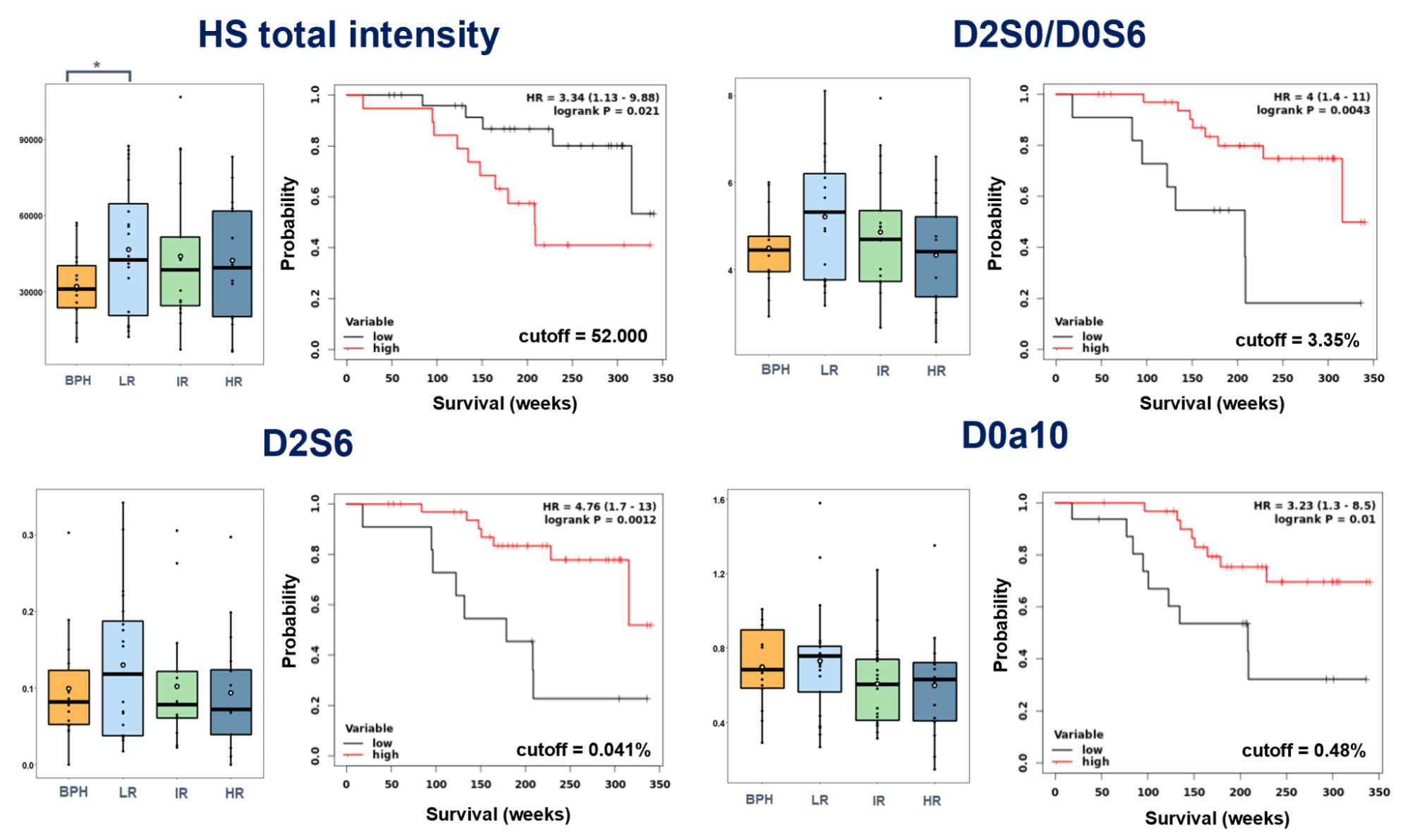
Figure 5. Correlations of GAG motif quantities with currently used risk assessment. Selected motifs include those that showed significant differences in survival along with their associated Kaplan-Meier curves.
Identification of independent markers
The observations made during the KM analysis suggested that it was worthwhile to examine the independence of markers showing significant differences in survival. For this, multivariate analysis was used on the clinical parameters (clinical tumor stage, PSA blood level, Gleason score) and the given GAG motif. I found that the D2S0/D0S6 and the D2S6 disaccharides are independent of clinical parameters (q <0.05 after Benjamini-Hochberg FDR correction), so their introduction into a future clinical practice could increase the accuracy of the survival risk assessment.
Expected impact and further research
The correlations shown in chondroitin sulfate studies indicate potential new therapeutic targets that can be targeted to make the biological therapy of prostate cancer more effective. Independent markers detected in heparan sulfate studies may open the way to new risk assessment methodologies for making therapeutic decisions in prostate cancer patients more efficient. However, this requires validation of the detected results in a large number of cases (n>1000). In case of successful validation, we plan to conduct studies to determine whether the detected markers can be measured by non-invasive sampling (e.g. urine), so that they can be easily implemented in clinical practice.
Publications, references, links
IF: impact factor
Strongly corresponding publications
[S1] Lilla Turiák, Gábor Tóth, Oliver Ozohanics, Ágnes Révész, András Ács, Károly Vékey, Joseph Zaia, László Drahos, Sensitive method for glycosaminoglycan analysis of tissue sections, Journal of Chromatography A, 2018, IF: 3.858
[S2] Lilla Turiák, Oliver Ozohanics, Gábor Tóth, András Ács, Ágnes Révész, Károly Vékey, András Telekes, László Drahos, High sensitivity proteomics of prostate cancer tissue microarrays to discriminate between healthy and cancerous tissue, Journal of Proteomics, 2018, IF: 3.509
[S3] Tóth Gábor, Vékey Károly, Drahos László, Turiák Lilla, A proteoglikánok és műszeres analitikai vizsgálatuk, Magyar Kémikusok Lapja, 2019, IF: -
[S4] Gábor Tóth, Károly Vékey, László Drahos, Viola Horváth, Lilla Turiák, Salt and solvent effects in the microscale chromatographic separation of heparan sulfate disaccharides, Journal of Chromatography A, 2020, IF: 4.759
[S5] Gábor Tóth, Károly Vékey, Simon Sugár, Ilona Kovalszky, László Drahos, Lilla Turiák, Salt gradient chromatographic separation of chondroitin sulfate disaccharides, Journal of Chromatography A, 2020, IF: 4.759
[S6] Simon Sugár, Gábor Tóth, Fanni Bugyi, Károly Vékey, Katalin Karászi, László Drahos, Lilla Turiák, Alterations in protein expression and site-specific N-glycosylation of prostate cancer tissues, Scientific Reports, 2021, IF: 4.379
[S7] Tóth Gábor, A rákkutatás jövője? – Fehérjék és cukrok vizsgálata mikrometszeteken, Élet és Tudomány, 2021, IF: -
[S8] Gábor Tóth, Domonkos Pál, Károly Vékey, László Drahos, Lilla Turiák, Stability and recovery issues concerning chondroitin sulfate disaccharide analysis, Analytical and Bioanalytical Chemistry, 2021, IF: 4.142
Loosely corresponding publications
[S9] Lilla Turiák, Simon Sugár, András Ács, Gábor Tóth, Ágnes Gömöry, András Telekes, Károly Vékey, László Drahos, Site-specific N-glycosylation of HeLa cell glycoproteins, Scientific Reports, 2019, IF: 3.998
[S10] Gábor Tóth, Fanni Bugyi, Simon Sugár, Goran Mitulović, Károly Vékey, Lilla Turiák, László Drahos, Selective TiO2 Phosphopeptide Enrichment of Complex Samples in the Nanogram Range, Separations, 2020, IF: 2.777
[S11] Andrea Reszegi, Katalin Karászi, Gábor Tóth, Kristóf Rada, Lóránd Váncza, Lilla Turiák, Zsuzsa Schaff, András Kiss, László Szilák, Gábor Szabó, Gábor Petővári, Anna Sebestyén, Katalin Dezső, Eszter Regős, Péter Tátrai, Kornélia Baghy, Ilona Kovalszky, Overexpression of Human Syndecan-1 Protects against the Diethylnitrosamine-Induced Hepatocarcinogenesis in Mice, Cancers, 2021, IF: 6.860
[S12] Gábor Tóth, Domonkos Pál, Simon Sugár, Ilona Kovalszky, Katalin Dezső, Gitta Schlosser, László Drahos, Lilla Turiák, Expression of glycosaminoglycans in cirrhotic liver and hepatocellular carcinoma – A pilot study including etiology, Analytical and Bioanalytical Chemistry, 2022, IF: 4.142
Other publications
[S13] Simon Sugár, Fanni Bugyi, Gábor Tóth, Judit Pápay, Ilona Kovalszky, Tamás Tornóczky, László Drahos, Lilla Turiák, Proteomic analysis of lung cancer types – a pilot study, Cancers, 2022, IF: 6.860
[S14] Dr. Róbert István Agócs, Dr. Domonkos Pap, Dániel András Sugár, Gábor Tóth, Lilla Turiák, Dr. Zoltán Veréb, Prof. Lajos Kemény, Prof. Tivadar Tulassay, Dr. Adam Vannay, Prof. Attila Jozsef Szabo, Cyclooxygenase-2 Modulates Glycosaminoglycan Production in the Skin during Salt Overload, Frontiers in Physiology, 2020, IF: 4.566
[S15] Gábor Tóth, Tanja Panić-Janković, Goran Mitulović, Pillar array columns for peptide separations in nanoscale reversed-phase chromatography, Journal of Chromatography A, 2019, IF: 4.049
[S16] Ádám Pálvölgyi, Zsolt Rapi, Oliver Ozohanics, Gábor Tóth, György Keglevich, Péter Bakó, Synthesis of alkyl a- and b-D-glucopyranoside-based chiral crown ethers and their application as enantioselective phase-transfer catalysts, Research on Chemical Intermediates, 2018, IF: 2.064
[S17] Zsolt Rapi, Oliver Ozohanics, Gábor Tóth, Péter Bakó, Lajos Höfler, Tamás Nemcsok, Nándor Kánya, György Keglevich, Syntheses and complexing ability of α-d-gluco- and α-d-xylofuranoside-based lariat ethers, Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2016, IF: 1.095
Table of links.
● MS Proteomics Research Group
● Tóth Gábor: A daganatterápiák kulcsa - fehérjék és szénhidrátok vizsgálata (in Hungarian)
● Proteoglikánok és műszeres analitikai vizsgálatuk (in Hungarian)
List of references.
[H1] A.K. Singh, R. Kumar, A.K. Pandey, Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers, Curr Chem Gen Translat Med. vol. 12 (2018). pp. 9–26.
[H2] Ahrens TD, Bang-Christensen SR, Jørgensen AM, Løppke C, Spliid CB, Sand NT, Clausen TM, Salanti A, Agerbæk MØ, The Role of Proteoglycans in Cancer Metastasis and Circulating Tumor Cell Analysis. Front. Cell Dev. Biol. (2020) 8:749.
[H3] A. Pudełko, G. Wisowski, K. Olczyk, E.M. Koźma, The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression, and metastasis of cancer, The FEBS Journal. vol. 286 (2019). pp. 1815–1837.
[H4] G.K. Tan, Y. Tabata, Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-κB nuclear translocation, Acta biomaterialia. vol. 10 (2014). pp. 2684–2692.
