|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
Faculty of Chemical Technology and Biotechnology, Department of Physical Chemistry and Materials Science
Supervisor: Dr. Gyarmati Benjámin
Mucoadhesion for more effective drug delivery
Introducing the research area
With mucoadhesive dosage forms that adhere to the mucosa (Figure 1) both systematic and local effects can be achieved with high bioavailability and patient compliance [1–3]. Despite some of these being already on the market, their characterization is not standardized, and the key factors determining the adhesion on mucosa are incomplete [4–6]. My PhD work aims to identify the critical aspects of the process of mucoadhesion and to design a polymer-based mucosa mimetic hydrogel that helps standardize the measurement of adhesion.
Figure 1. Tesa Mucofilm® a buccal mucoadhesive film.
Brief introduction of the research place
Soft Matters group has a significant experience in the synthesis and characterization of polymers and gels [7–9]. In the current work, we can build on this knowledge to understand the process of mucoadhesion across various size scales. We synthesize polymers with unique chemical structures to study and understand the process of adhesion on a molecular level.
History and context of the research
In the design of a drug delivery system, the most important aspect besides safety is to achieve absorption and reach the desired effect systematically or locally at the site of the application. If a systematic effect is desired, oral (per os) administration is the most common. In this case, the drug is absorbed from the small intestine or the stomach and enters the first-pass metabolism. As a result, a significant amount of the drug can be eliminated, which decreases bioavailability, moreover, may also lead to side effects.
In contrast to conventional oral administration, transmucosal drug delivery provides drug release directly into the systematic circulation, which means higher bioavailability and a decreased chance of side effects to be developed [1–6]. A dosage form used for transmucosal drug delivery can be a buccal adhesive polymer film (e.g., Belbucca®) or tablet (e.g., Fentora®) for managing chronic, long-lasting pain. Also, in the case of a vaginal contraceptive film (e.g., VCF®) which adheres to the inner wall of the vagina, the exact mechanism of adhesion is considered.
Nevertheless, exploiting the phenomenon of mucoadhesion is not straightforward as long-lasting, adequately strong adhesion should be achieved on mucosa: a soft surface covered with mucus bearing high water content is highly challenging [1, 4–6], since the biological role of the mucosa, besides lubrication, is to eliminate pathogens, dirt and foreign substances from our body.
The research goals, open questions
For the rational design of mucoadhesive dosage forms, the process of mucoadhesion must be known [4–6]. My aim is to determine the key factors affecting the strength and length of adhesion. Currently, there are only some poorly defined theories in the literature (Figure 2) that give some clues to the critical factors during adhesion, however, they do not provide information on the relative importance of each of these factors in the formation and preservation of the mucoadhesion for various mucus membranes and dosage forms.
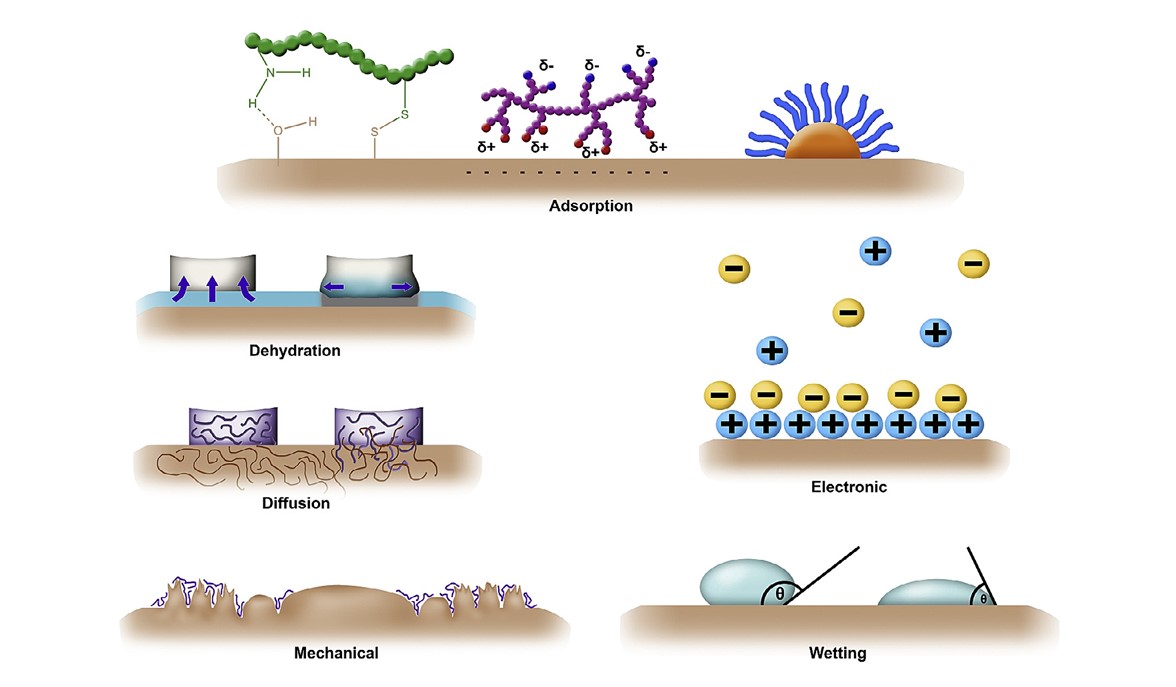
Figure 2. Theories of mucoadhesion [12].
One of the main reasons for the poor understanding of mucoadhesion is associated with its typical measurement. It is usually measured ex-vivo, using animal tissues that differ significantly partly due to the natural biodiversity and also as a consequence of dissimilarities during preparation and storage. These differences result in a random error during the measurement [5, 6]. To reduce these random errors, a robust, well-defined mucosa mimetic material is needed that mimics the adhesive properties of the mucosal surface [5,6,10]. With such a mucosa-mimetic material, we will be able to determine the critical factors in different cases. I also aim to explore the molecular and colloidal processes behind macroscopic adhesion to gain a deeper understanding, since without attractive interfacial interactions, adhesion is not possible. To this end, I am exploring the attractive interfacial interactions leading to adhesion [4–6].
Methods
To untangle the process of mucoadhesion, I propose a bottom-up approach, that is, starting from the molecular scale and studying the molecular interactions at first. Molecular interactions can be analyzed with mucin glycoprotein. Mucin is the main component of the mucus covering the mucosal membrane (Figure 3), although it can also be found on the mucosal membrane in transmembrane, membrane-bound form. Its structure resembles a bottlebrush, causing oligosaccharide side chains to attach to the main chain of the mucin protein. At the end of these sidechains, usually, there is a sialic acid unit (pKa ~ 2.7) that gives a negative surface charge to the glycoprotein. Besides carboxylic acid functionality, thiol groups can also be found on the mucin in the cysteine-rich regions. In addition to the primary structure of mucin, it is essential that these mucin subunits can form a crosslinked structure that (nearly) keeps its shape by covalent bonds (disulfide bridges) or by hydrophobic associations [4–6, 11,13]. All these structural features must be considered when designing mucoadhesive formulations, and polymer structures should be tailored according to their possible attractive interactions with mucin.
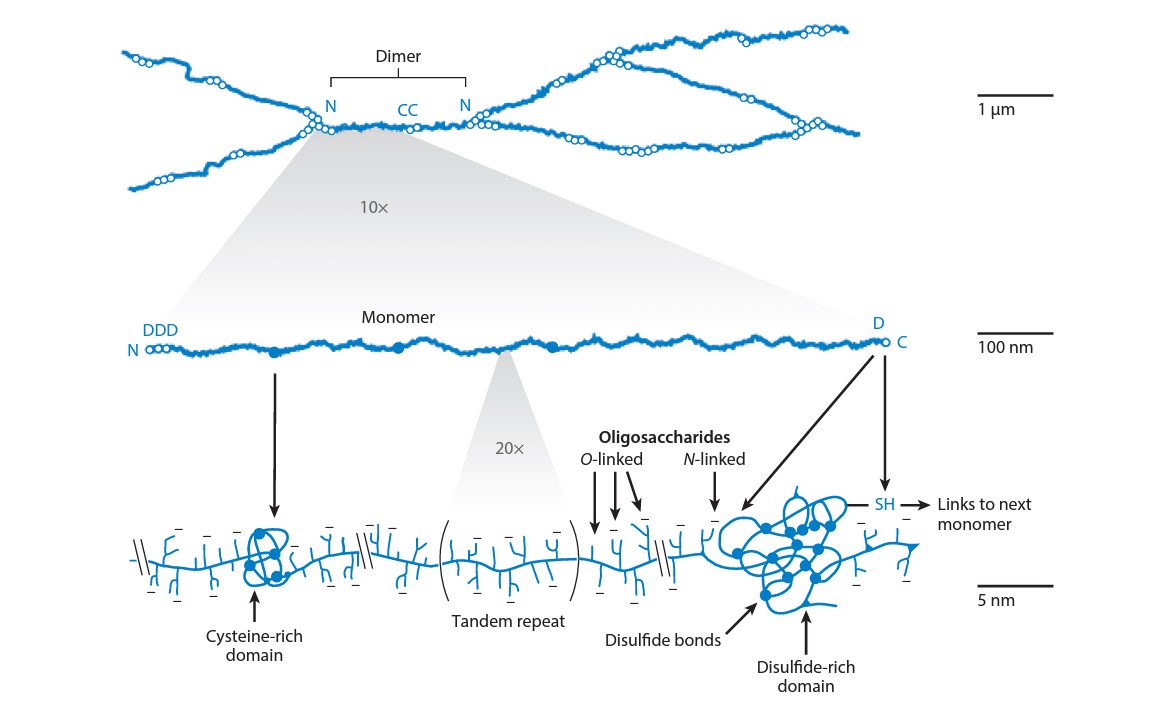
Figure 3. Structure of the mucin glycoprotein [13].
In my work, I use commercially available polymers and polymers synthesized by myself. From the commercial ones, I use polyacrylic acid (Carbopol®) chitosan hydroxypropyl-methylcellulose (HPMC) because of their different ionic nature (negatively, positively charged, and neutral respectively). My polymers are polyaspartamides, which can bear various side chains according to the modifying agents and their concentration used [SG 2,3]. The resulting polyaspartamides can be anionic, cationic, or neutral; moreover, they can be thiolated or have a hydrophobic nature.
I use isothermal titration calorimetry (ITC) to characterize molecular interactions of the polymers. With ITC, the enthalpy and equilibrium constant of the process can be described quantitatively. Currently, I use a negatively charged poly(amino acid) as a model of mucin. On the colloidal length scale, I study the adsorption of polymers on a thin mucin layer on quartz crystal microbalance (QCM) (Figure 4). With this method, I can quantify how many micrograms of polymer are bound on mucin per weight, and after adsorption, I can also study the desorption caused by shear forces characteristic of the human body. In the colloidal methods, I use mucin and study the formation of particles upon polymer addition due to attractive interactions between the polymer and the mucin. These results complement those obtained from thin layers (QCM). Colloidal changes can be followed through the change in apparent absorbance with UV-Vis spectrometry (turbidimetry), with the particle size analysis by dynamic light scattering (DLS), and by measuring the surface charge by zeta potential measurement.
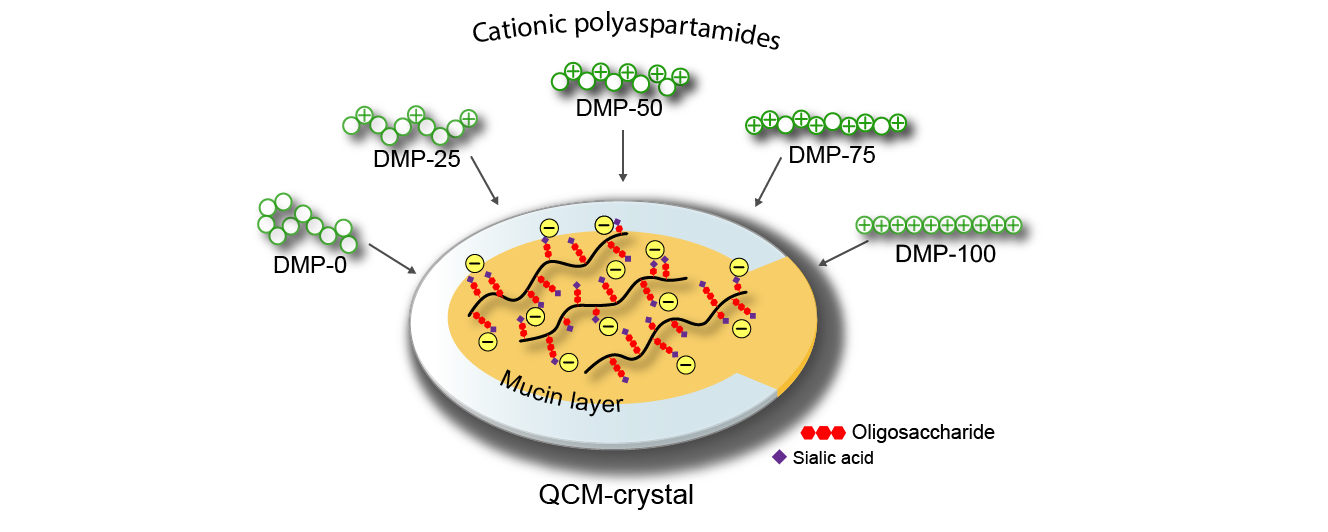
Figure 4. Adsorption of polyaspartamides with different cationic side group content on thin layer of mucin adsorbed on a QCM crystal [SG2].
To measure adhesion on a macroscopic size scale (Figure 5), I will press tablets from my polymers; with the tablets attached to a mechanical tester, a measurement sequence is applied. First, the tablet is brought into contact with the substrate (mucosa mimetic material). Second, the contact force is maintained for a specific time, and finally, the tablet is slowly detached from the surface by pulling. Throughout the measurement, the force is measured as a function of the distance from which the work and the force of adhesion are determined. For this kind of measurement, I designed polymer-based mucosa mimetic hydrogels. The viscoelastic characteristics of those hydrogels are analyzed by oscillatory rheology that determines the energy dissipation of the gel, which is a key factor in adhesion. Based on the results, the gel structure can be further optimized to mimic natural mucosa better.
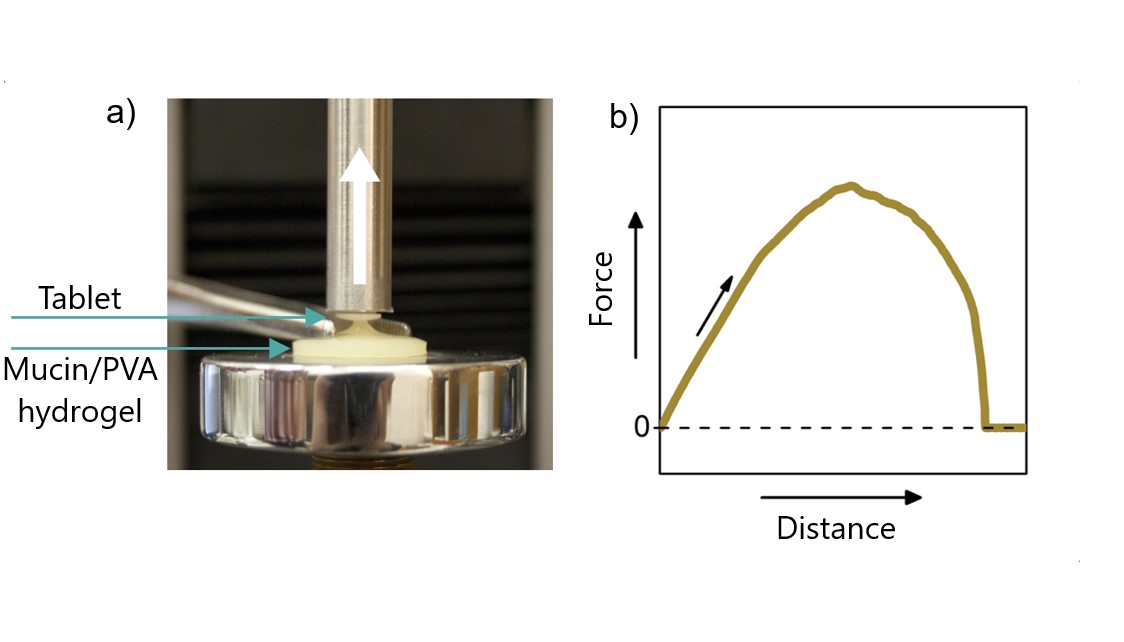
Figure 5. Study of adhesion on macroscopic level using a Mucin/PVA hydrogel substrate developed by me a) demonstration of adhesion with a tablet (not the complete measurement setup) b) an adhesion curve from the measurement [SG1].
Results
Utilizing the quartz crystal microbalance (QCM) technique, I explored a non-linear polymer structure-property relationship between the cationic side group content of polyaspartamides and their adsorption on the mucin layer (Figure 6). Polyaspartamides with medium (50-75%) cationic content showed the highest adsorption. In the future, the polymer structure can be designed accordingly, and other functional groups (e.g., thiols) also can be incorporated that can form additional bonds with mucin glycoprotein, further improving the strength of mucoadhesion potentially increasing the retention time of the dosage form on the mucosa.
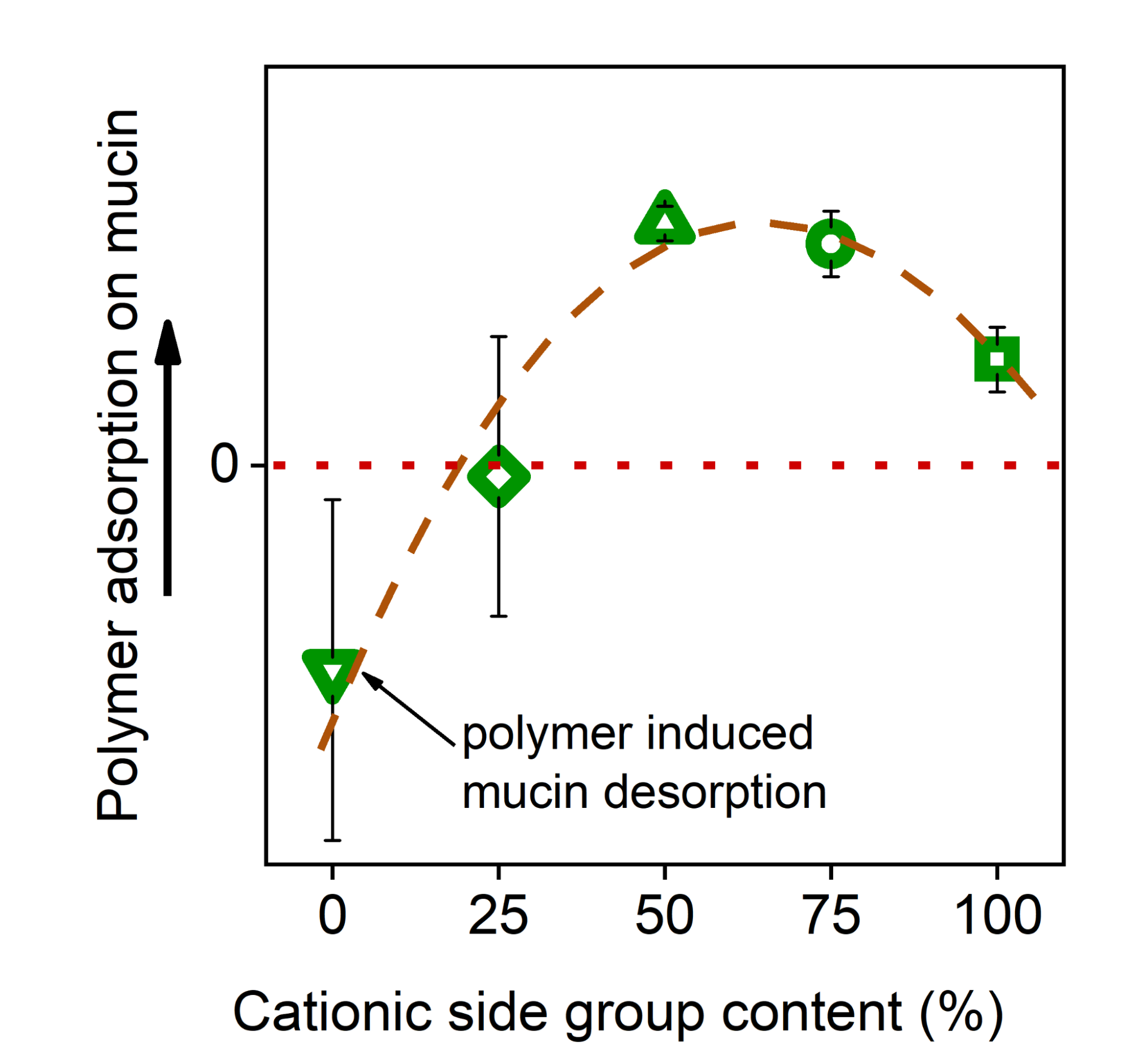
Figure 6. Adsorption of cationic polyaspartamides on mucin as a function of cationic side group content [SG2].
Based on my DLS turbidimetry and zeta potential results, I proposed a theory for mucin-polymer interaction resulting in particle formation. The model describes the building and the change in particle structure, number, and size upon mucin-polymer interaction as a function of concentration. This theory helps us understand the results gained from colloidal, high throughput measurements which can accelerate the screening of mucoadhesive performance of the new polymers.
For the study of mucoadhesion on the macroscopic scale, I designed a robust mucosa mimetic mucin-containing hydrogel. The mucin protein was entrapped into the poly(vinyl alcohol) network containing only -OH groups. The gelation was performed using freezing-thawing gelation, without chemical crosslinking to preserve the structure of the glycoprotein. On this mucosa-mimetic material, the adhesion measurement of well-known adhesive tablets was accomplished with excellent reproducibility. Polyacrylic acid (Carbopol®) showed significantly higher adhesion than hydroxypropyl methylcellulose which showed modest adhesion, likewise, determined ex-vivo, in the literature previously. Carbopol might show higher adhesion due to its slightly crosslinked structure and its polyanionic character resulting in repulsive interactions with mucin and. Thus, a high affinity for water develops great osmotic pressure that might promote the interpenetration of polymer chains (Figure 7).
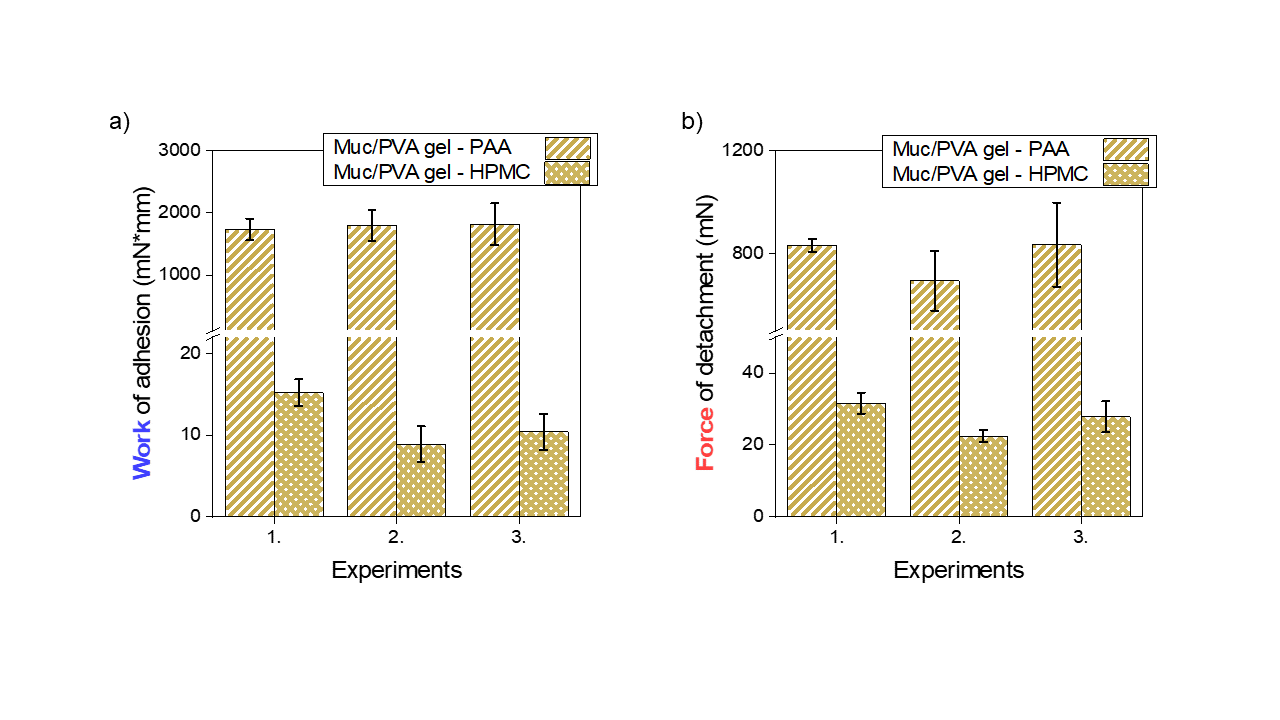
Figure 7. Results of adhesion measurements on the mucosa mimetic Mucin/PVA hydrogel for polyacrylic acid (PAA, Carbopol®) and hydroxypropyl-methylcellulose (HPMC) tablets a) works of adhesion b) force of detachment [SG1].
I proved that the mucin in my hydrogel-based mucosa mimetic material affects adhesion through electrostatic interactions (Figure 8). In the case of the mucin-containing gel, higher adhesion was measured for cationic chitosan tablets compared to mucin-free PVA gels. In the case of anionic PAA, the relationship was reversed. No difference was found in the case of hydroxyethyl-methylcellulose. All these results verified the electrostatic theory of adhesion macroscopically.
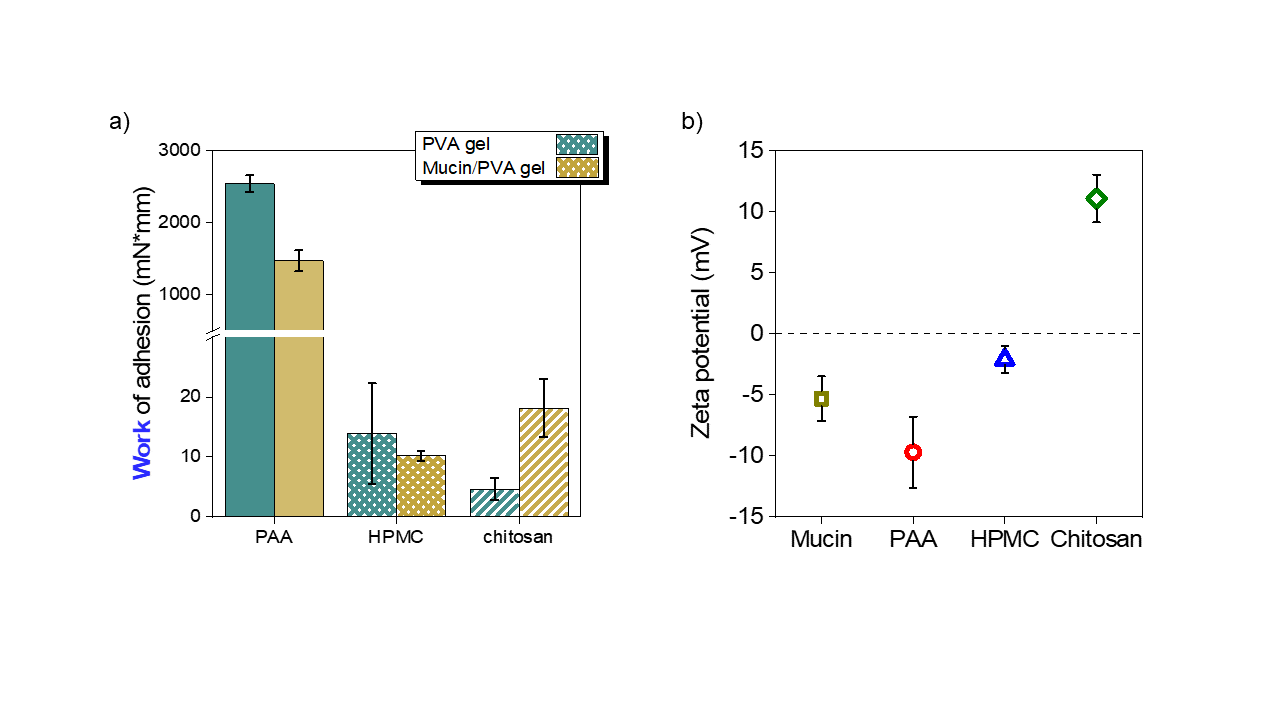
Figure 8. a) Work of adhesion measured on Mucin/PVA hydrogel for polyacrylic acid (PAA), hydroxypropyl-methylcellulose (HPMC) and for chitosan tablets b) zeta potentials for the different polymers [SG1].
Expected impact and further research
The importance of the work is shown by the fact that the first main publication of my PhD work on the synthesis of the mucosa mimetic hydrogel was published in a high-impact Q1 journal (Colloids and Bionterfaces B IF = 5.999) in 2022 and has already received one independent citation. In my recent work, I studied the adsorption of cationic polyaspartamides with different cationic group content on a thin mucin layer. This manuscript is currently under review at the International Journal of Biological Macromolecules (Q1, IF= 8.025). In my future work, I will focus on the hierarchical nature of the adhesion to better link the experience gained at the molecular and supramolecular levels. These results can be applied in the medium term in the engineering of new mucoadhesive dosage forms.
Publications, references, links
List of corresponding own publications. (IF: impact factor)
[SG1] Gyarmati B, Stankovits G, Szilágyi BÁ, Galata DL, Gordon P, Szilágyi A. A robust mucin-containing poly(vinyl alcohol) hydrogel model for the in vitro characterization of mucoadhesion of solid dosage forms Colloids and Surfaces B: Biointerfaces 2022, 213: 112406 (IF 2022 = 5.999)
[SG2] Stankovits G, Ábrahám Á, Kiss É, Varga Z, Mirsa A, Szilágyi A, Gyarmati B, The interaction between mucin and poly(amino acid)s with controlled cationic group content in bulk phase and in thin layers International Journal of Biological Macromolecules, 2023, under review (IF 2022 = 8.025)
[SG3] Gyarmati B, Mammadova A, Barczikai D, Stankovits G, Misra A, Alavijeh MS, et al. Side group ratio as a novel means to tune the hydrolytic degradation of thiolated and disulfide cross-linked polyaspartamides. Polymer Degradation and Stability, 2021,188: 109577 (IF 2021 = 5.030)
[SG4] Gyarmati B, Mammadova A, Stankovits G, Barczikai D, Szilágyi A. Effect of Side Groups on the Hydrolytic Stability of Thiolated and Disulfide Cross-linked Polyaspartamides Periodica Polytechnica Chemical Engineering 2021, 65: 183–191 (IF 2021 = 1.571)
Table of links.
List of references.
[1] Gottnek M., Hódi K., ifj. Regdon G. Gyógyszerészet 2013, 57: 69–75
[2] Beg S, Swain S, Rizwan M, Irfanuddin M, Shobha Malini D. Bioavailability Enhancement Strategies: Basics, Formulation Approaches and Regulatory Considerations. Current Drug Delivery 2011: 8: 691–702
[3] Park K. Controlled drug delivery systems: Past forward and future back. Journal of Controlled Release 2014, 190: 3–8
[4] Bayer IS. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies Advanced Materials Interfaces 2022, 9: 2200211
[5] Khutoryanskiy VV. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromolecular Bioscience 2011, 11: 748–64.
[6] Smart JD. The basics and underlying mechanisms of mucoadhesion. Advanced Drug Delivery Reviews 2005, 57: 1556–68.
[7] Gyarmati B, Mammadova A, Barczikai D, Stankovits G, Misra A, Alavijeh MS, et al. Side group ratio as a novel means to tune the hydrolytic degradation of thiolated and disulfide cross-linked polyaspartamides. Polymer Degradation and Stability 2021, 188: 109577
[8] Gyarmati B, Vajna B, Némethy Á, László K, Szilágyi A. Redox- and pH-responsive cysteamine-modified poly(aspartic acid) showing a reversible sol–gel transition. Macromolecular Bioscience 2013, 13(5): 633–640
[9] Gyenes T, Torma V, Gyarmati B, Zrínyi M. Synthesis and swelling properties of novel pH-sensitive poly (aspartic acid) gels. Acta Biomater. 2008, 4(3): 733–744.
[10] Cook MT, Khutoryanskiy VV. Mucoadhesion and mucosa-mimetic materials—A mini-review. International Journal of Pharmaceutics Volume 2015, 495 (2): 991–998
[11] Navarro LA, French DL and Zauscher S, Advances in mucin mimic synthesis and applications in surface science Current Opinion in Colloid & Interface Science 2018,
38: 122–134
[12] Cook SL, Bull SB, Methven L, Parker JK, Khutoryanskiy VV Food Hydrocolloids, Mucoadhesion: A food perspective 2017, 72: 281–296
[13] Carlson TL, Lock JY and Carrier RL Engineering the Mucus Barrier Annual Review of Biomedical Engineering 2018, 20: 197–220
