|
|
BMe Research Grant |
|
George A. Olah Doctoral School of Chemistry and Chemical Technology
BME VBK, Department of Applied Biotechnology and Food Science
Supervisor: Dr. VÉRTESSY Beáta, Dr. TASNÁDI Gábor
Biocatalytic asymmetric synthesis of amines by reductive amination
Introducing the research area
The overall aim of the PhD project is the development and application of a modern synthesis method based on natural catalysts (enzymes) for the production of compounds of pharmaceutical interest, mainly chiral amines. The industrial relevance of the research is routed in the large number of chiral amine structural elements in drug molecules, the production of which represents a significant synthetic challenge. This research will result in an enzyme collection and the associated knowledge base that will enable the environmentally friendly and efficient production of chiral amines.
Brief introduction of the research place
The research is conducted in collaboration between the Biostruct Laboratory of the Budapest University of Technology and Economics and the Servier Research Institute of Medicinal Chemistry. With its outstanding expertise in structural biology, the Biostruct Laboratory allows the bioinformatic identification and analysis of novel enzyme sequences, protein production, and purification by fermentation, as well as structure modeling and elucidation. As an industrial partner, the Servier Research Institute offers a high-standard infrastructure for the testing of commercial enzyme panels and in-house enzymes, the synthesis of target substrates, the development of analytical methods, and the testing of synthetic applications.
History and context of the research
The structure of modern drug candidate molecules is increasingly complex, often containing multiple chirality centers, and the economical synthesis of these molecules is a huge challenge for synthetic chemists. Therefore, technologies that reduce the number of synthetic steps, and increase their selectivity and overall efficiency are of increasing importance in process development. Biocatalysis can make a major contribution to these goals, thanks to the sustainable production and use of enzymes and their exceptional selectivity (Figure 1). Biocatalysis has evolved tremendously over the last two decades from a laboratory method to an industrially relevant technology. This progress is mainly due to the designability and engineerability of enzymes, driven by the explosion of biological sciences and the directed evolution technology. Whereas industrial enzymatic processes used to be severely limited by the need to tailor the process parameters to the properties of the enzyme, nowadays in many cases the enzyme is tailored to the process conditions and substrate(s) (enzyme engineering). As a result, an increasing number of industrial enzymatic processes are being developed, many of them at production scale[1].

Figure 1 - Advantages of biocatalysis in industrial manufacturing processes.
It is estimated that about 40% of pharmaceuticals contain a chiral amine building block, therefore, there is great interest in producing them in enantiomerically pure form. Several enzymatic approaches for the synthesis of chiral amines are known, with several new families of enzymes recently being introduced. A subgroup of imine reductases (IREDs), the so-called reductive aminases (RedAms), have been shown to catalyze imine formation, thus achieving high conversions with the use of near stoichiometric amounts of amine partners[2]. This type of reactivity allows the coupling of two molecules (carbonyl and amine) to yield chiral secondary amine compounds. With suitable substrates, members of other less-explored enzyme families, such as ketimine reductases (KIRED) or opine dehydrogenases (ODH), also show reductive aminase activity[3].
The research goals, open questions
This research aims to generate and functionally characterize a reductive-aminase enzyme collection. The new enzyme panels will be composed partly of known enzymes and partly of new enzymes identified by bioinformatic methods.
The primary target reaction is the coupling of various amino acid derivatives via the amine group with a variety of carbonyl compounds, mainly ketoacids (Figure 2). The resulting chiral secondary amine dicarboxylic acid derivatives are analogs of naturally occurring opine compounds[4], which can be converted into further derivatives via side-chain functional groups or by modification of the carboxylic acid function. They can be valuable building blocks for bioactive molecules such as peptidomimetics. Examples of such compounds are the ACE2 inhibitors shown in Figure 3.
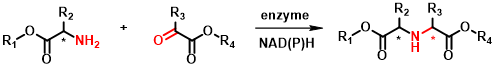
Figure 2 - General equation of the target reductive amination reaction of this research.
Adaptation to the unique building blocks of a drug molecule requires knowledge of the enzyme's binding site and active site residues. Using this information, we can make modifications to the enzyme’s structure to enable the synthesis of diverse chiral amine compounds with high stereoselectivity. The research also aims at the computer-aided design of focused variant libraries and the development of a method for the rapid and simple production and testing of the designed variants. This will allow the generated enzyme panels to be rapidly extended and customized to solve a given synthetic problem.
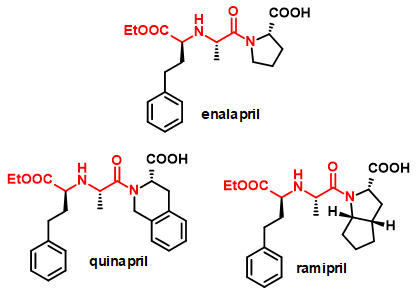
Figure 3 - Drug molecules containing chiral secondary amine dicarboxylic acid building blocks.
With enzymes selected from the enzyme collection that most efficiently convert the model substrates, we also aim to optimize the reaction conditions to achieve preparative-scale transformations. This scale-up step is of particular importance for the industrial application of these new enzymatic processes.
Methods
The research takes a comprehensive approach to building a reductive-aminase enzyme collection using multiple approaches. The most obvious approach is to test enzymes previously described in the literature with model substrates. By exploiting the substrate tolerance of enzymes, the range of molecules that can be efficiently transformed by a given enzyme can be extended. This approach will be used to test commercially available enzymes, such as the imine reductase (IRED) collection from the UK company Prozomix[5], as well as known members of other less explored enzyme families, such as ketimine reductases (KIRED) and opine dehydrogenases (ODH). The expandability of an enzyme’s substrate scope is usually limited, and additional methods are needed to solve a given synthetic problem. If an enzyme shows insufficient activity on a substrate, two solutions are available (Figure 4).
The first is to exploit the natural diversity of enzymes (biodiversity). Sequence similarity-based bioinformatic methods can be used to find new enzymes in genomic and metagenomic databases that have similar activity but a different substrate scope. These latter databases can also be a source of enzymes with particularly valuable properties, as they often contain genetic information from samples from extreme environments[6]. The proteins encoded in them are more tolerant to the non-natural conditions (high temperatures, organic solvents, etc.) encountered in industrial applications. In this project, new sequences are identified from environmental metagenomes by academic collaborators, and the encoded enzymes are produced and characterized.
The second option is to improve the activity of the enzyme by modifying its amino acid sequence (enzyme engineering). This approach is used in the Nobel Prize-winning method of directed evolution, whereby iterative rounds of massive mutagenesis produce an enzyme with significantly increased activity[7]. However, this technology is very time- and resource-intensive. If information is available on the structure of the enzyme and its mechanism of action, mutations can be rationally designed to improve activity, thus significantly reducing the number of variants to be tested[8]. In this research, computationally designed mutations will be applied to selected enzymes to increase their activity or change their substrate preference.
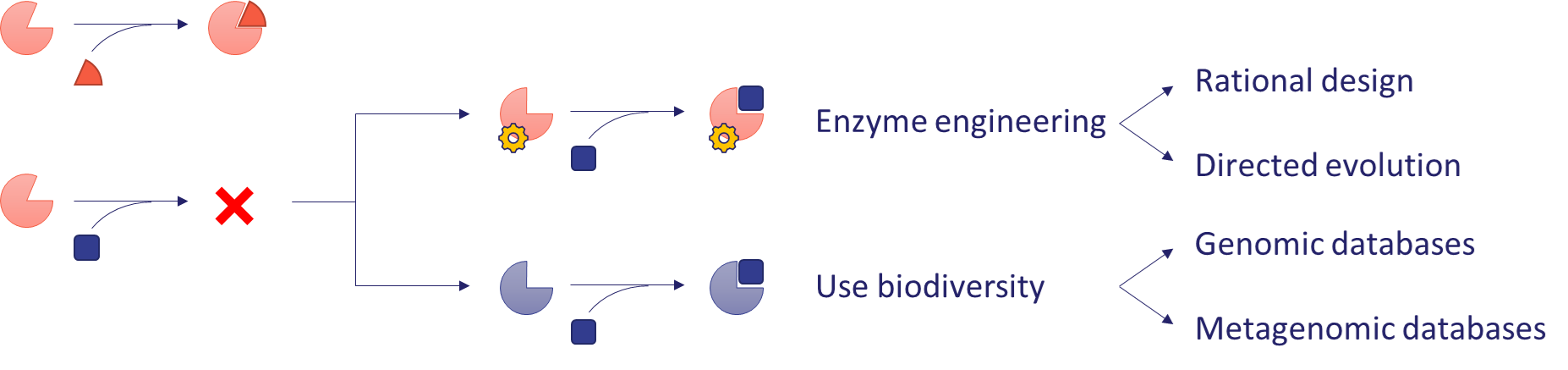
Figure 4 - Strategies to come up with an enzyme that efficiently transforms a novel substrate.
However, one mutation rarely results in a sufficient increase in activity, so testing of smaller variant libraries is still necessary even in the case of rational design. In this research, we are also developing a method that is suitable for the rapid production and testing of medium-sized (from 10s to 100s) variant libraries. The limiting factor in this process is the bacterial expression and purification of the modified enzymes. To speed up this step, we use a cell-free protein expression system. Using this method, the small amount of enzyme required for initial activity tests can be produced quickly and easily, greatly speeding up the process[9]. For the cell-free expression system to be suitable for the testing of enzyme variants, we are developing an innovative, new method for the cell-free (purely PCR-based) production of variant DNA sequences. By combining this process with cell-free expression, the enzyme engineering required for industrial application can be realized quickly even in a chemistry laboratory.
Results
The IREDs were tested based on their reported activity on α-ketoesters, which allows the synthesis of N-substituted amino acid derivatives[10]. In the course of our research, we have extended the range of described substrates with α-ketoacid and amino ester-type molecules (Figure 5). After testing several substrate combinations, 18 enzymes were selected from the 384-member collection, which were also tested in purified form and the reaction conditions were optimized.
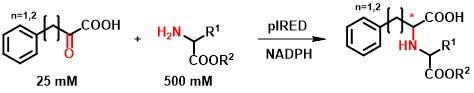
Figure 5 - New reactivity of IREDs.
KIREDs and ODHs can perform reductive amination of α-keto acids, the former with primary amines[11] and the latter with α-amino acids[12]. In a study of 3 literature-known KIRED enzymes, we found that the enzyme family exhibits outstanding activity with α-ketoacid/α-aminoester substrate combinations (Figure 6A). We conducted more detailed studies with the best-performing enzyme (RnKIRED from Rattus norvegicus) and explored its substrate scope in detail (Figure 6B). As part of this process, the reaction conditions (pH, temperature, substrate concentration, enzyme concentration) have been optimized and the absolute configuration of the resulting products is currently being determined. An interesting observation was that the size of the substrates influences the configuration of the newly formed stereocenter: in the reaction between a large ketoacid and a small aminoester, RnKIRED shows S-selectivity, whereas, in the reaction between a small ketoacid and a large aminoester, it displays R-selectivity (Figure 6C).
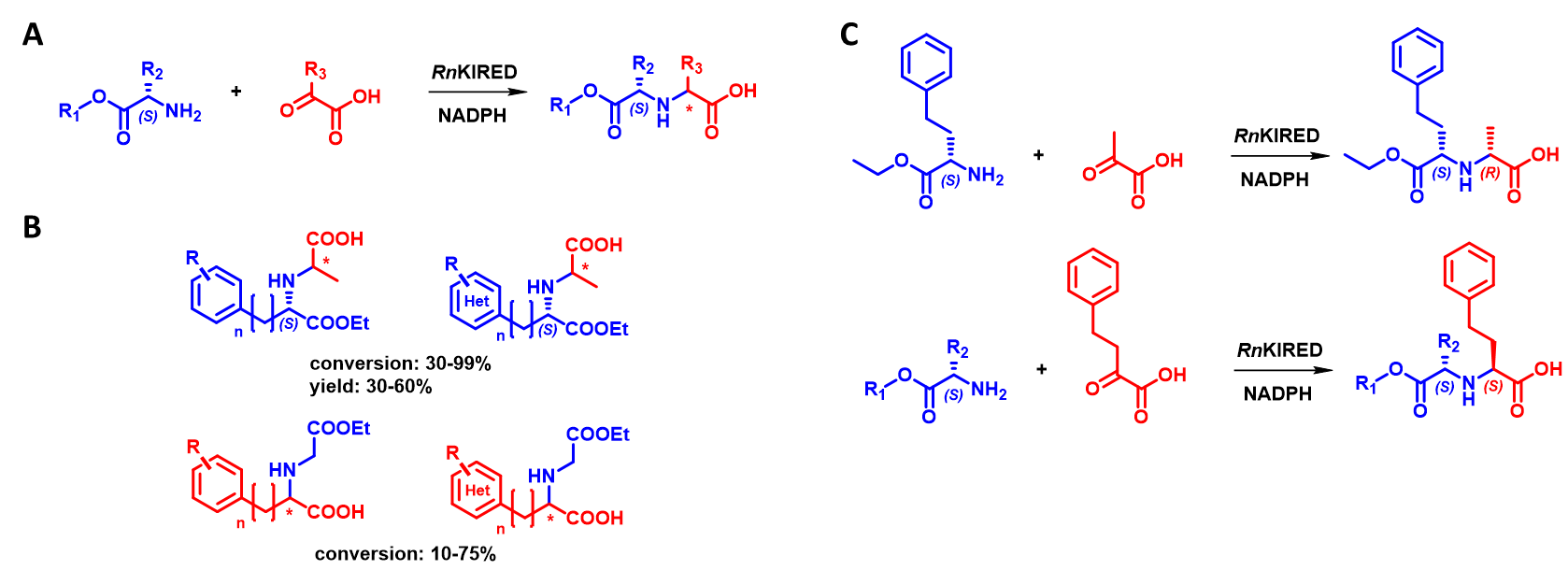
Figure 6 - A General scheme of the new reaction catalyzed by RnKIRED.
B Substrate scope of RnKIRED
C Switch of stereoselectivity of RnKIRED based on substrate sizes.
The 5 known ODH enzymes we synthesized and tested showed activity only in the described ketoacid/aminoacid combination, not with ketoester or aminoester. However, this less-explored family of enzymes was further investigated, as the reaction they catalyze is very close to the defined target reaction. In collaboration, we have identified 10 new ODH sequences from a hotspring metagenome and characterized 6 of them. These completely new metagenomic opine dehydrogenases (mODHs) were found to be more thermotolerant than ODHs previously reported. The mODHs showed different activity on substrates compared to those described in the literature, typically being able to convert negatively charged polar amino acids. A typical substrate was L-aspartic acid (Figure 7B), but they also accepted non-canonical amino acids such as L-cysteic acid. Several preparative size (~100 mg) transformations were performed with a selected metagenomic enzyme. As a result, three highly functionalized chiral secondary amine derivatives were produced and chemically characterized (Figure 7C).
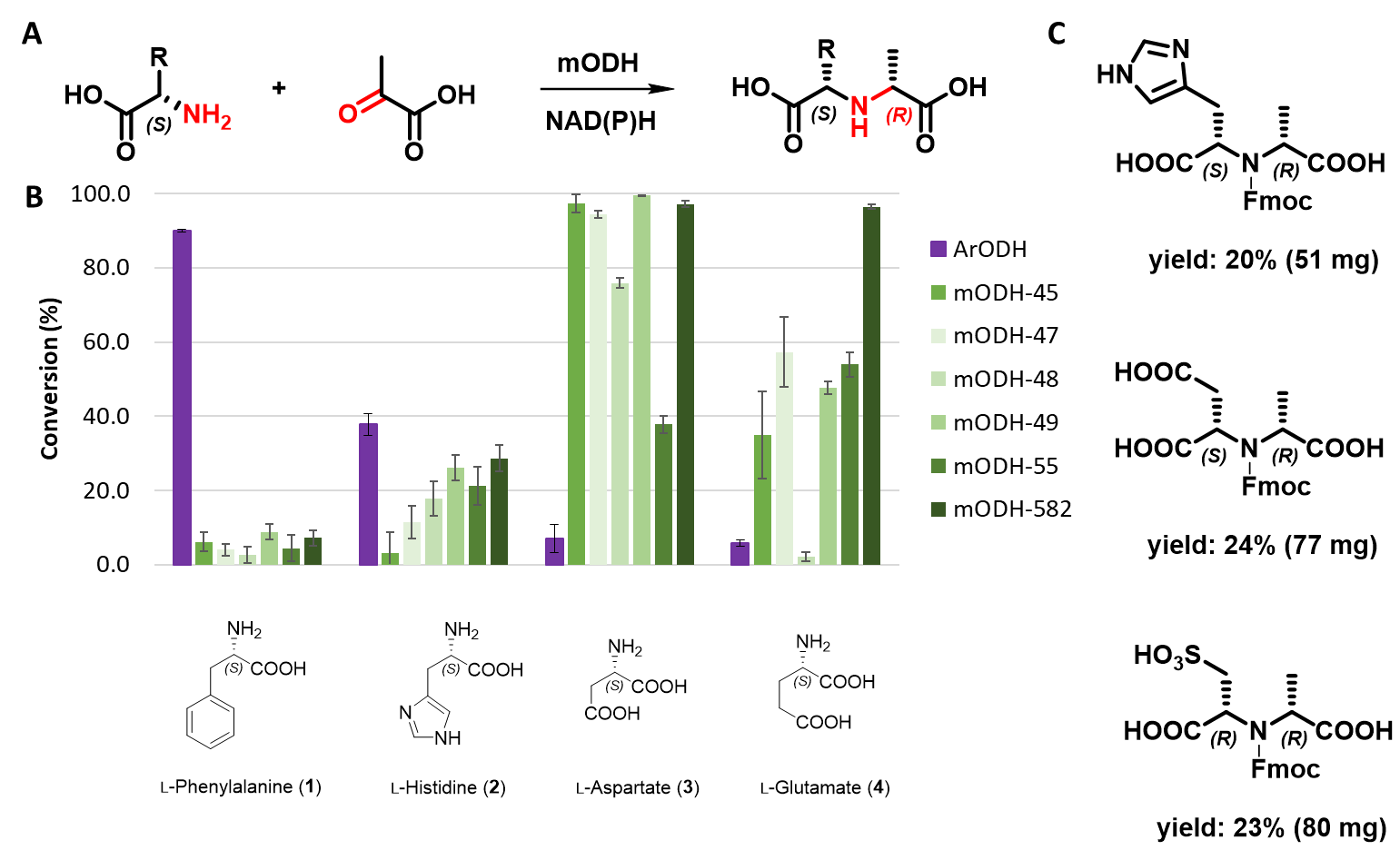
Figure 7 - A General scheme of the reaction catalyzed by mODHs. B Substrate preference of mODHs compared to the reference ArODH. C Compounds synthesized on a preparative scale using mODHs.
The unprecedented amino acid preference of the mODHs has been explained by structural models. A more detailed study of the active site has revealed that the chemical character of some amino acid side chains, as well as their spatial proximity, may be crucial. Based on this, we have modified two positions in the active pocket, which reversed the substrate preference of the ODH enzymes: the double mutant version of the metagenomic enzyme accepted apolar amino acids, while the double mutant version of the reference enzyme transformed negatively charged polar amino acids (Figure 8).
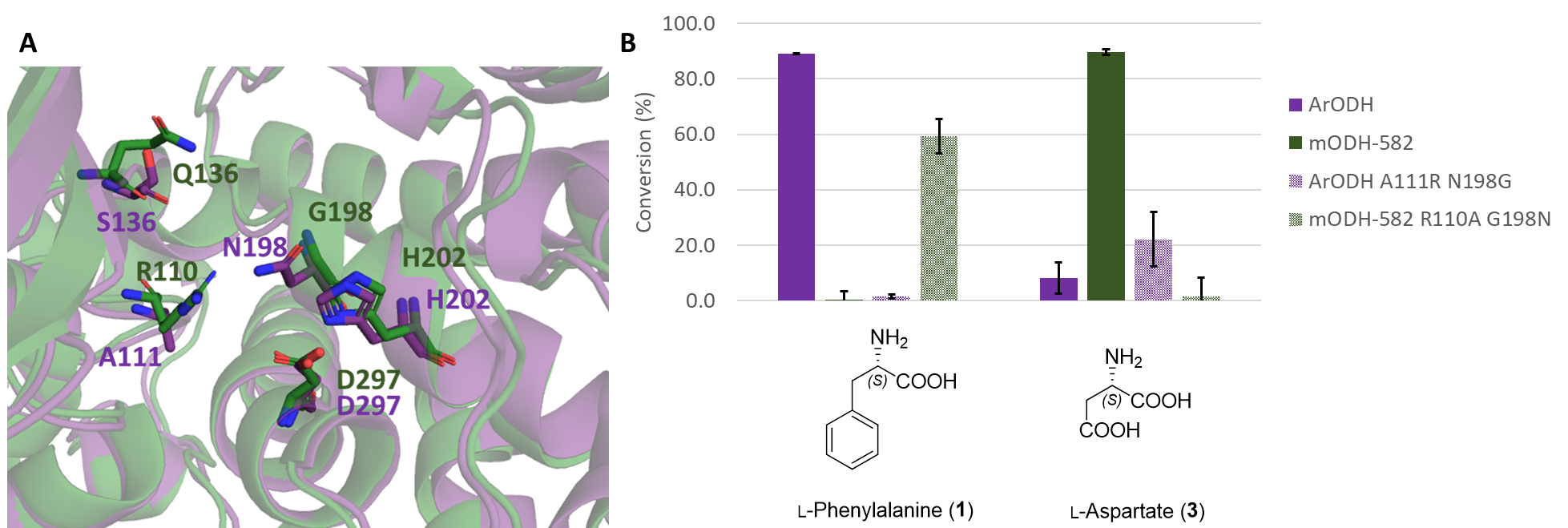
Figure 8 - A Comparison of the active site of mODHs (green) and ArODH (purple), key amino acid side chains are displayed. B Switch of substrate preference of ODHs as a result of rationally designed double mutations.
In this project, we have also successfully established a cell-free protein expression system that enables the production and purification of small amounts of an enzyme using linear template DNA generated by PCR. This allows rapid testing of new enzymes or enzyme variants. An innovative method for the cell-free production of variant DNA sequences was developed and validated on ODH and KIRED mutants. In the latter case, a computationally designed 60-member variant library was also successfully produced.
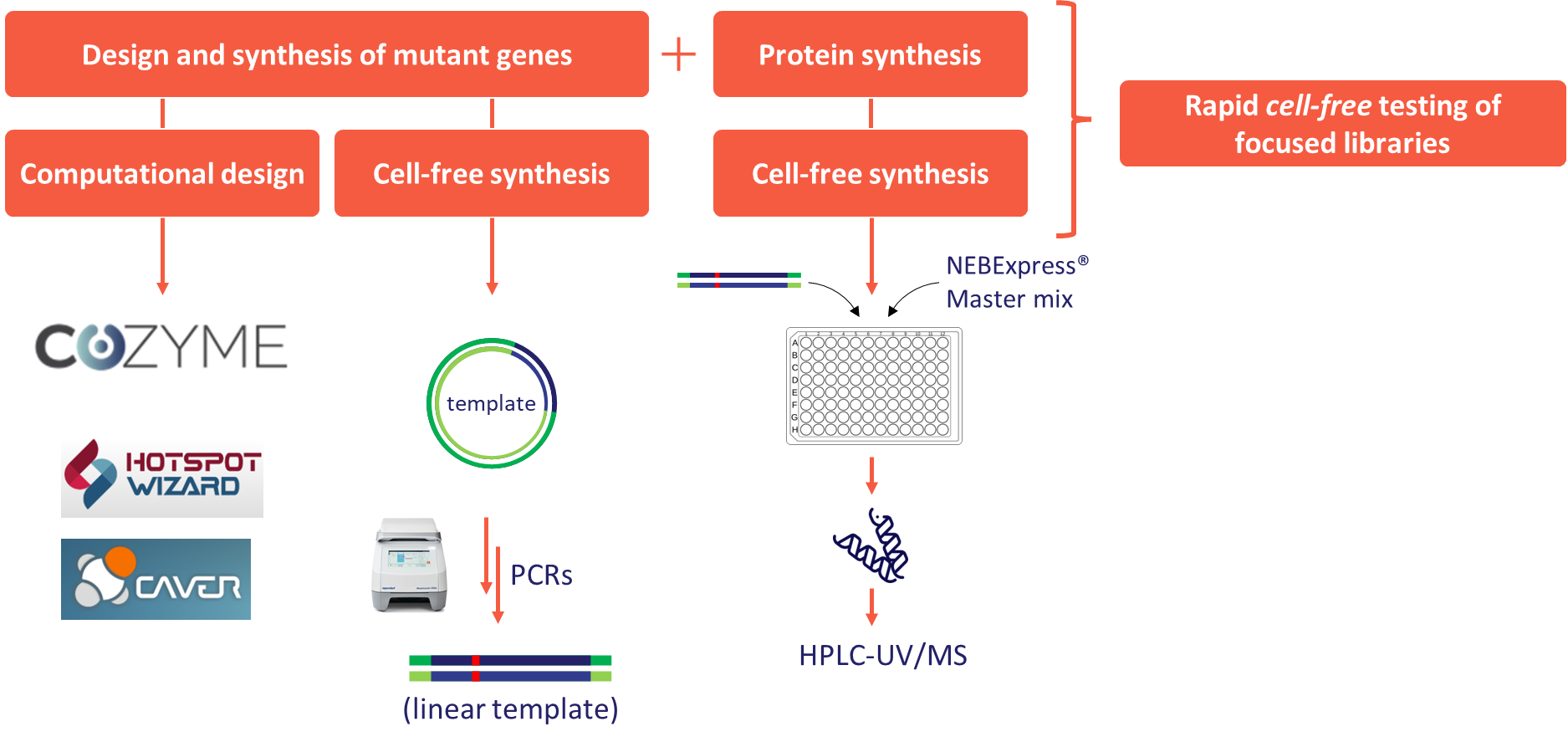
Figure 9 - Concept of the cell-free enzyme engineering workflow.
Expected impact and further research
The research so far has yielded significant results in the development of enzymatic processes for reductive amination with all three different approaches. Our results have a significant impact on the biocatalytic activities at Servier and the extension of the related infrastructure. We have extended the scope of the IRED and KIRED enzyme families with new substrates. We have expanded the ODH enzyme family with new members from metagenomic sources, characterized these enzymes in detail, and demonstrated their synthetic utility. The cell-free mutagenesis and expression method we have developed also allows for in-house engineering of the already available enzymes (Figure 9). We also aim to extend this method to the study of new enzyme families (amine dehydrogenases) and the engineering of the RnKIRED enzyme. The latter development aims at the stereoselective production of a key intermediate in ACE2 inhibitor drugs.
Publications, references
List of corresponding own publications:
András Telek, Zsófia Molnár, Beáta G Vértessy, Gábor Tasnádi: Opine dehydrogenases, an underexplored enzyme family for the enzymatic synthesis of chiral amines. Biotechnol. Bioeng., 2023, 120, 2793–2808.
András Telek, Zsófia Molnár, Kristóf Takács, Bálint Varga, Vince Grolmusz, Gábor Tasnádi, Beáta G Vértessy: Discovery and biocatalytic characterization of opine dehydrogenases by metagenome mining. Appl. Microbiol. Biotechnol. 2024, 108, 1–16.
List of references:
[1] E. L. Bell et al. Nat. Rev. Methods Primers 2021, 1, 46−67.
[2] M. D. Patil et al. ACS Catal. 2018, 8, 10985−11015.
[3] J. H. Schrittwieser et al. Adv. Synth. Catal. 2015, 357, 1655–1685.
[4] J.F. Hyslop et al. J Biotechnol. 2019, 293, 56–65.
[5] J. R. Marshall et al. Nat. Chem. 2021, 13, 140−148.
[6] S. L. Robinson, J. Piel, S. Sunagawa Nat. Prod. Rep. 2021, 38, 1994–2023.
[7] J. L. Porter, R. A. Rusli, D. L. Ollis ChemBioChem 2016, 17, 197–203.
[8] A. Phintha, P. Chaiyen Chem. Catal. 2022, 2, 2614–2643.
[9] J. Rolf, K. Rosenthal, S. Lütz Catalysts 2019, 9, 190–207.
[10] P. Yao et al. Angew. Chemie Int. Ed. 2021, 60, 8717–8721.
[11] J. F. Hyslop et al. Angew. Chemie 2018, 130, 14017–14020.
[12] Y. Kato, H. Yamada, Y. Asano J. Mol. Catal. B Enzym. 1996, 1, 151–160.
Project no. C1580174 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development, and Innovation Fund, financed under the NVKDP-2021 funding scheme.
