
|
BMe Research Grant |

|
Oláh György Doctoral School (BME)
Department of Physical Chemistry and Material Science
Supervisor: Krisztina László
Metal Doped Carbon Gels
Introducing the research area
Carbon aerogels are constructed from covalently bonded nanoparticles. Owing to their special properties (extremely low density, high surface area and porosity, unusual combination of high electrical and low thermal conductivity, good IR absorption) the application potential of these materials is very wide. Their morphology and pore size distribution are tunable via the preparation, and they can be made in different shapes. The principal application fields are: supercapacitor electrodes (104 F/g, 77 F/cm3), adsorbents (approximately 50% porosity), membranes (tunable pore size), catalysts/catalyst supports and solar cells.
My aim was to prepare and characterize carbon gels containing transition metals (copper and titanium) that can be used as membranes (copper) for gas separation or for photocatalytic applications (TiO2).
Brief introduction of the research place
The scientific activity of the Surface Chemistry Group of the Department of Physical Chemistry and Materials Science focuses on
-
preparation, characterization, modification of nanoporous systems;
-
investigation of solid/liquid interface phenomena;
-
application of rigid and flexible gels in sorption processes;
-
surface characterization by gas and liquid adsorption.
History and context of the research
The first organic precursor of carbon aerogels
was obtained from the Na2CO3 catalyzed sol-gel
reaction of resorcinol and formaldehyde in aqueous medium [1]. The three-dimensional hydrogel matrix resulting from this
polycondensation was transformed into a solid carbon skeleton (carbon gel)
(Figure 1).
Organic aerogels are brittle and harder than their
inorganic counterparts. The extremely high thermal resistance (six times greater
than glass wool) makes them potential insulators. Their outstanding features are
the high surface area and pore structure. They can be obtained in various forms,
from microspheres to binder-free monoliths (tens of centimeters).
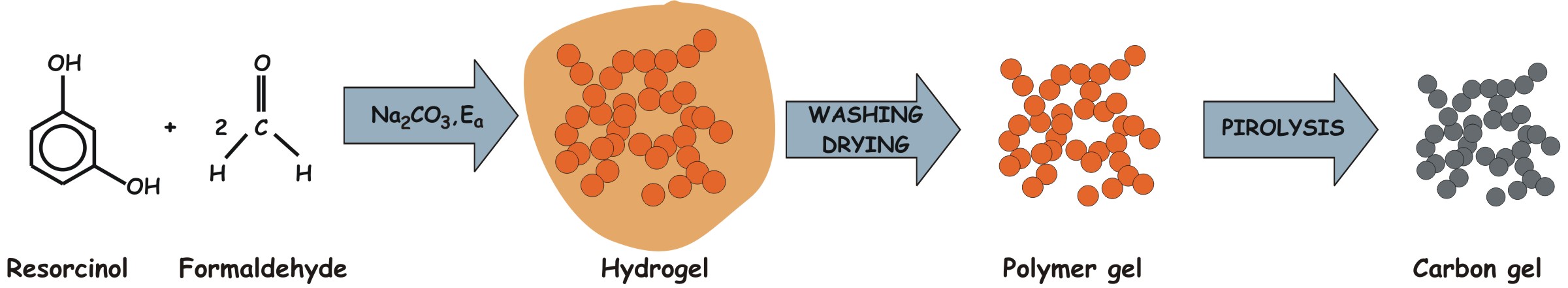
Figure 1: Preparation of carbon
gels
Tuning of the pore structure and the surface area
can be achieved by changing the initial conditions (stoichiometry, catalyst,
concentration, solvent, temperature) or by the drying process (simple heat
treatment, freeze-drying, supercritical extraction) and finally by the
carbonization parameters (duration, atmosphere, temperature).
Metal doping
may enhance the performance of the carbon gels. The synthesis route of RF gels
has the advantage that metals can be introduced at several different stages.
With appropriate transition metals, specific properties, such as membrane
selectivity or catalytic activity, can be developed and/or improved.
Aim of the research
-
Study of the morphological differences caused by the different drying methods:
Supercritical CO2 (scCO2) drying is the most widely used process to obtain mesoporous RF gels, but no systematic studies of the effect of the conditions were reported previously. The supercritical extraction referred in the literature means an extraction step with liquid CO2, and CO2 is transformed to the supercritical state just before the depressurizing step. As the extraction capacity of scCO2 is generally better than that of liqCO2, my intention was to study the parameters of the real supercritical conditions. -
Preparation of copper and titania containing carbon gels:
Copper containing carbon gel can be used for separating CO/CO2 gas mixtures, while TiO2 is a well-known photocatalyst in the decomposition of aromatic compounds.
I intended to study the possibility of doping with copper(II) and titania(IV) salts and their effect on the structure of the carbon gels. The metals were introduced into the systems at three different stages: -
during polymerization (1st step)
-
impregnation of the dry polymer aerogel (2nd step)
-
impregnation of the carbon gel (3rd step).
Methods
Synthesis
The metal-free RF hydrogel
was prepared [2] in accordance with the recipe of Lin [3]. The one-week gelation process was performed at 85 °C. For
doping, copper-acetate [4] and titanium-isopropoxide [5] were used as metal sources. Prior to drying, the water in
the pores of the hydrogels was exchanged for acetone (or t-butanol, in the case
of freeze-drying).
The effects of the concentration and the anion of the
impregnating solutions on the metal uptake were studied independently on
microporous carbons.
Drying
To obtain dry polymer gels from the hydrogel,
three methods were employed:
-
Heat-treatment [2] in nitrogen atmosphere, resulting in xerogels.
-
Freeze-drying [2] at -45 °C to prepare cryogels.
-
Aerogels were prepared with drying by CO2 extraction. Samples were obtained either by the method described in the literature [2] or using true supercritical conditions and continuous CO2 flow throughout the whole procedure. In the latter case the pressure, temperature and the duration of the drying were studied systematically.
Carbonization
The dried polymer gels were
converted to carbon gels in a rotary quartz-reactor, in a one-hour long heat
treatment at 900 °C, under nitrogen atmosphere.
Characterization
-
Dynamic light scattering (DLS) measurements were made in the sol–gel reaction bath, using an ALV goniometer (ALV, Langen, Germany). A HeNe laser (632.8 nm) was used as the light-source, with an ALV 5000E multi-tau correlator. To avoid multiple scattering, the samples were placed in 1 or 2 mm diameter capillaries. Measurements were performed in the temperature range 30–50 °C.
-
Surface morphological investigations were made on a JEOL 5500 (JEOL, Japan) scanning electronmicroscope (SEM). The polymer samples were coated with Au/Pd to prevent charging. Elemental analysis of the samples was performed by the EDS function of the apparatus, in high vacuum mode. The average metal concentration was calculated from at least six measurements.
-
Small and wide angle X-Ray scattering (SAXS/WAXS) measurements were made on the BM2 small angle camera in the European Synchrotron Radiation Facility (ESRF), Grenoble, France. An indirect illumination CCD detector was used and the powdered samples were placed in glass capillaries of diameter 1.5 mm.
-
The specific surface area and pore volume of the samples were determined from nitrogen adsorption/desorption measurements performed at -196 °C with an Autosorb-1 (Quantachrome, USA) computer controlled volumetric gas adsorption apparatus.
-
The photocatalytic activity of the titania-doped samples was tested with phenol-decomposition measurements.
Results
Effect of drying on
the morphology of the carbon gels
The effects of drying method on the carbon gel morphology were studied. SEM images (Figure 2) illustrate differences arising during drying. These persist after carbonization, yielding carbon xero-, cryo- and aerogels with significantly different morphologies.
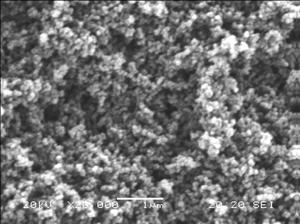
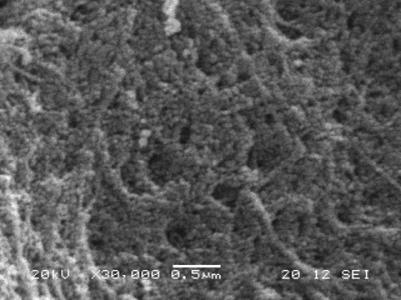

(a) (b) (c)
Figure 2: Electron microscopy images of carbon xerogel (a),
cryogel (b) and aerogel (c) in 20 000 or 30 000-times
magnification
Xerogels are the most compact, with the smallest specific surface area (110
m2/g). Cryogels display the loosest structure and highest surface
area (570 m2/g). Liquid CO2 extraction yields intermediate
morphological properties (surface area: 270 m2/g). The porosity and
size of the building blocks depend on the drying method. On carbonization, the
basic units shrink, except in the xerogel, where the elementary units coalesce.
Apparent surface areas increase: carbon xerogels have typically ~900
m2/g, cryogels ~2500 m2/g and aerogels ~1000
m2/g.
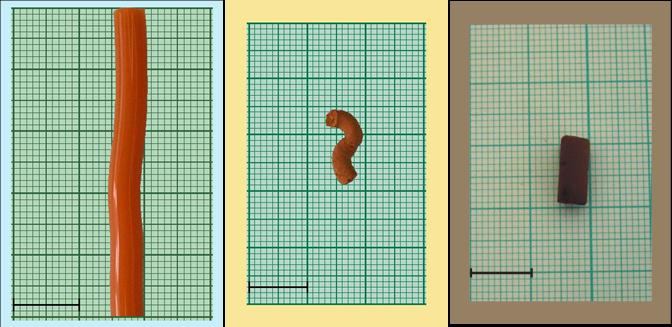
(a)
(b)
(c)
Figure 3: Appearance of the hydrogel (a), aerogel dried with
liquid (b) and supercritical (c) CO2
(Scale bar: 1
cm)
Significant differences arise between the liquid
and supercritical CO2 dried samples (Figure 3). The latter
display loose, mainly macroporous structures of high surface area comparable to
the freeze-dried sample. During extraction with liquid CO2 the
building spheres coalesce, forming units 3-4 times bigger than with
supercritical CO2. The parameters of true supercritical drying affect
mainly the macropore content of the samples.
Effect of
transition metals on the structure of the polymer and carbon
aerogels
Although the copper is not incorporated into the
polymer matrix, its presence during the polymerization results in significantly
smaller elementary beads and an outstandingly high apparent surface area (almost
2000 m2/g) in the polymer aerogel state. The apparent surface area
and pore size distribution (Figure 4) of the carbon gels can be tuned
according to the copper doping technique [6]. This can enhance the selectivity of the carbons in gas
separation processes. This effect was not observed in the titania-doped
samples.
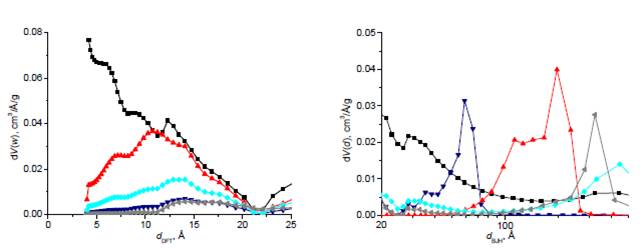
(a)
(b)
Figure 4: Size distribution of micro (a) and mesopores
(b) of copper containing carbon gels (■–metal-free
carbon gel, carbon gels doped in the 1st (▲), 2nd (▼) and 3rd (low copper content:◆,
high copper content:◄) steps
Heat treatment after introduction of the metals
changes the oxidation state of the copper and the crystallinity of the titanium
compound. Copper(II) reduces to metallic copper clusters, while the originally
amorphous TiO2 becomes a mixture of crystalline anatase and rutile,
imparting photocatalytic activity to the samples.
Expected impact and further research
Owing to the controllable pore structure and the flexible and relatively easy means of doping, carbon gels have attracted a great interest. In the future I will focus on membrane and catalyst applications and I intend to perform a detailed study of the polymerization reaction in the presence of metals in order to improve doping efficiency. The doping experiments will be extended to molybdenum because its chemical properties designate it as a replacement for chromium-containing catalysts, thus replacing the carcinogenic chromium. The gas separation properties and liquid state catalytic performance of the samples will be also tested.
Publications, references, links
Publications:
a) K. László: O. Czakkel, B. Nagy, E.
Geissler: Resorcinol formaldehyde gels for advanced carbon
materials
Intl. Symp. on Modern Problems of Surface
Chemistry and Physics, 18–21 May 2010, Kiev, Ukraine, Programme and Abstract Book pp. 81–82. (ISBN 978-966-02-5619-4)
b) O. Czakkel, E. Geissler, I. M. Szilágyi, E. Székely, K. László: Cu-doped resorcinol-formaldehyde polymer and carbon aerogels, Journal of Colloid and Interface Science, 337 (2009) pp. 513–522
c) O. Czakkel, E. Geissler, A. Moussaïd, B.
Koczka, K. László: Copper-containing
resorcinol-formaldehyde networks
Microporous and
Mesoporous Materials, 126 (2009) pp. 213–221
d) E. Székely, O. Czakkel, B. Nagy, K.
László, B. Simándi: Aerogélek szárítása (Drying of Aerogels, in Hungarian)
Olaj Szappan Kozmetika, Különkiadás (2009) pp. 84–87
e) O. Czakkel, I. Szilágyi, E. Geissler, N.
Kanellopoulos, K. László: Morphological characterization of
oxidized and metal impregnated spherical carbons, Progress in Colloid and Polymer Science, 135 (2008)
pp. 139–147
f) O. Czakkel, K. Marthi, E. Geissler, K. László: Influence of drying on the morphology of resorcinol-formaldehyde-based carbon gels: Microporous and Mesoporous Materials, 86 (2005) pp. 124–133
Links:
Dynamic light scattering (DLS)
Small and wide angle X-Ray scattering (SAXS/WAXS)
European Synchrotron Radiation Facility
(ESRF)
[1] R.W. Pekala: Journal of Materials Science, 24 (1989) p. 3221
[2] O. Czakkel, K. Marthi,
E. Geissler, K. László: Microporous and Mesoporous Materials, 86
(2005) p. 124
[3] C.
Lin, J.A. Ritter: Carbon 35 (1997) p. 1271
[4] O. Czakkel, E.
Geissler, A. Moussaïd, B. Koczka, K. László: Microporous and Mesoporous
Materials, 126 (2009) p. 213
[5] F.J. Maldonado-Hodar, C. Moreno-Castilla, J. Rivera-Utrilla: Applied Catalysis A 203 (2000) p. 151
[6] O. Czakkel, E. Geissler, I.M. Szilágyi, E. Székely, K. László: Journal of Colloid and Interface Science, 337 (2009) p. 513
