 |
BMe Research Grant |

|
Faculty of Chemical Technology and Biotechnology
Department of Applied Biotechnology and Food Science/Fermentation Pilot Plant Laboratory
F-Labor
Introducing the research area
The research in F-labor focuses on the industrial
applications (fermentations) of microorganisms which  is the target area of biotechnological operations, at the same time. In these microbial fermentations
(carried out by bacteria, yeasts and other fungi as well as viruses) lots of
valuable (and marketable) products are formed during cell growth. The main task of our research group is the examination and optimization
of this product formation. During
the development of a fermentation technology, the primary goal is to quantify
and satisfy the demands of the microbes in order to obtain maximal productivity.
For this reason, our research usually starts on the lab scale by selecting the
appropriate producer strain, and then continues with the optimization of (1) nutrient
requirements (carbon, nitrogen sources, trace demand, vitamin demand etc.) and 2)
condition parameters: pH, temperature, aeration, fermentation technique etc.).
Finally, fermentation on various (pilot) scales can improve the
reliability of the developed process and serves as the basis for technology
transfer.
is the target area of biotechnological operations, at the same time. In these microbial fermentations
(carried out by bacteria, yeasts and other fungi as well as viruses) lots of
valuable (and marketable) products are formed during cell growth. The main task of our research group is the examination and optimization
of this product formation. During
the development of a fermentation technology, the primary goal is to quantify
and satisfy the demands of the microbes in order to obtain maximal productivity.
For this reason, our research usually starts on the lab scale by selecting the
appropriate producer strain, and then continues with the optimization of (1) nutrient
requirements (carbon, nitrogen sources, trace demand, vitamin demand etc.) and 2)
condition parameters: pH, temperature, aeration, fermentation technique etc.).
Finally, fermentation on various (pilot) scales can improve the
reliability of the developed process and serves as the basis for technology
transfer.
Brief introduction of the research place
The name "F-Labor" discloses much about the group (especially in Hungarian where all the concepts shown in the pictures below start with F):
 Building F |

Fermentation |
 Downstream and protein purification |
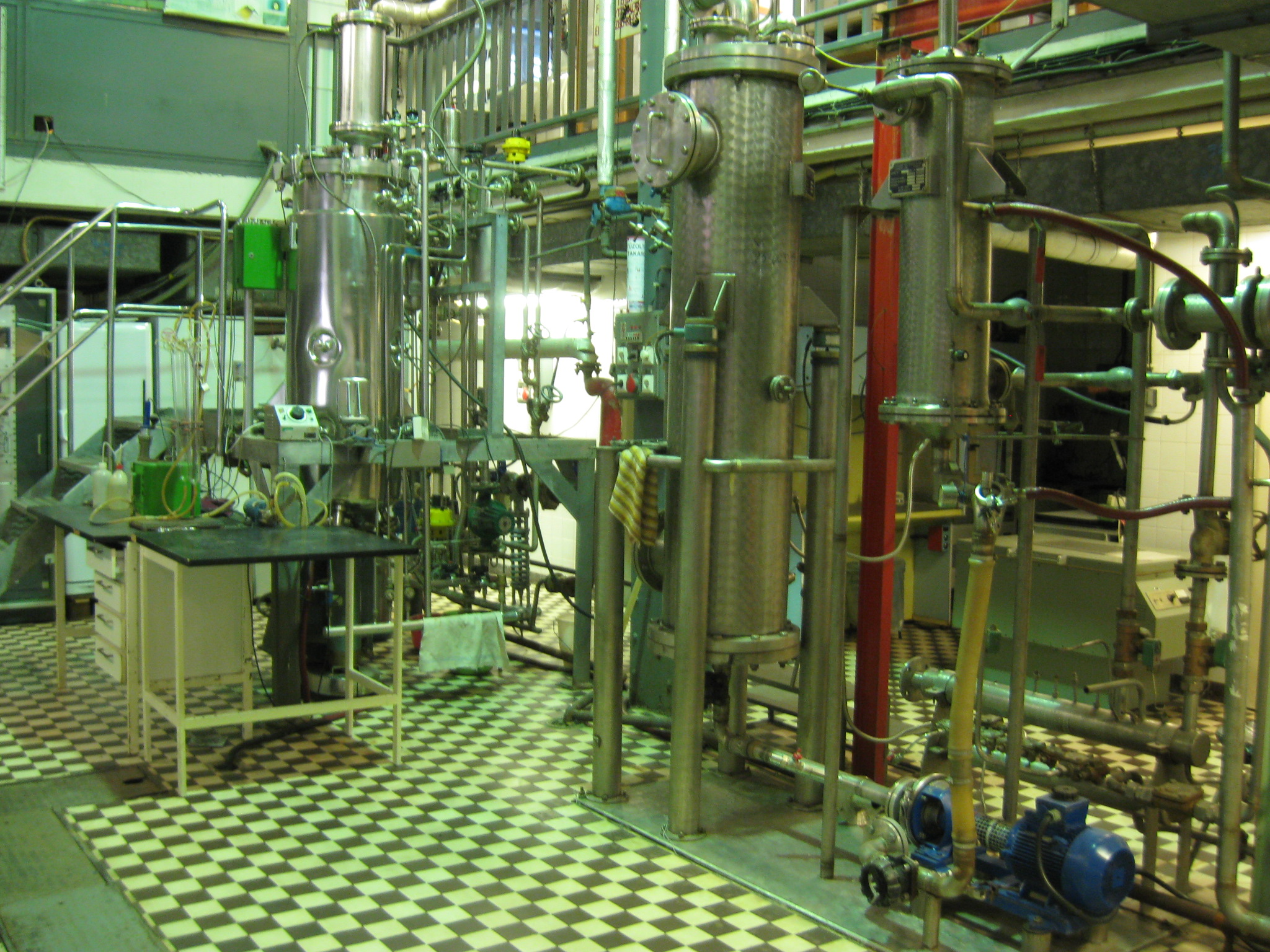 Pilot scale |
The Fermentation Pilot Plant research group was founded more than 25 years ago. Its recent infrastructure has been serving the operations for more than a decade now. Our infrastructure is used for industrial coworkers as well as educational and research purposes. The pilot scale facilities have established our potential to join the first Hungarian Biorefinery project, which examines the fermentative production possibilities of lactic acid.
History and context of the research
It was a strategic decision in the 80's in Hungary whether to improve or worn out the biotechnological plants still in operation at that time. Thanks to the strong commitment of the Bioengineering Working Group of HAS, they have been improved, and so our Fermentation Group was founded at BUTE Faculty of Chemical Engineering located at the then existing Department of Agricultural Chemical Technology, to fulfill the rising demand on pilot scale research. Developments in the frame of ongoing industrial requests and scientific and technological researches concluded in a modern fermentation pilot plant joining projects like the antibiotics production at Biogal Pharma or B12 fermentation in Richter Pharma or lysine (amino acid) production technology in Kaba.
If we distinguish areas of biotechnology by the widely accepted
color codes, the above-mentioned researches belong to Red
(Health and Pharma) Biotechnology. Although our research group have already
had cooperations in the field of Green (feed and food, and agriculture)
Biotechnology as well (for example to produce preparation against the Fire blight of the
apples and its relates), currently the most dominant researches are conducted in the field of White Biotechnology, primarily in 1,3-propandiol enzymatic production, and in fermentative lactic acid production. Both researches are involved in
the BUTE Research University project as topics of Researches for White Biotechnology methods
under the Biotechnology-Health and Environment protection priority research
area.
The aim of the white biotechnology branch is to "whiten" or clean the chemical
industry, that is to convert it into environmentally neutral form. (The goals
are partly the same as in green chemistry but the tools are significantly
different.)
The structure of the Chemical industry is strongly hierarchic with petroleum on the top as a single energy and raw material source. Through its refining, the first basic molecules can be obtained, from which the chemical reactions start in the industry. On the analogy of these refineries, the term "biorefinery" was introduced meaning first only the conversion of some renewables (like starch) into products, however, their 2nd and 3rd generation plants have the flexibility to adapt to different raw materials and market demands. In this way, the former hierarchy have been converted into a technological matrix.
From the renewable (plant origin) raw materials Biorefineries
produce so called "platform-forming" basic molecules, which are
equally valuable for the classic chemical industry. 1,3-propanediol and lactic
acid are both such platform chemicals. While the former can be used as a solvent
or as an anti-freezing agent but mostly as a monomer for the plastic industry,
lactic acid is widely used in food industry (preservative), for cosmetic (hydrating
agent) and pharma (biodegradable surgeon cord) purposes. From Poli-lactic acid
(PLA) biodegradable polymer can be produced, so it is one of the target
molecules in the packaging industry.
The research goal, open questions
The biological production of 1,3-propanediol was realized in parallel with our researches: the fermentation plant of Tate & Lyle started in 2006 as a result of an international cooperation, in which de novo recombinant Escherichia coli produces 40.000t/yr 1,3-propanediol. In the case of microbial fermentations, a given part of the raw material is converted into (cell)biomass, and decreases the product formation. In addition, usually certain metabolites are also created which is a further source of raw material loss. Considering these, we developed a coenzyme linked process on the labscale, in which the by-product of biodiesel (namely the glycerol, which is a renewable in this context) will be converted into 1,3-propanediol and oxidized coenzyme (NAD+). This oxidized coenzyme will simultaneously be back-reduced in a revert reaction, meanwhile another molecule of glycerol will be oxidized into 1,3-dihydroxyacetone (DHA), which is the active agent of self-tanning preparations in the cosmetic industry. In this way, a renewable waste can be converted in one simple step into two valuable industrial products, without by-products.
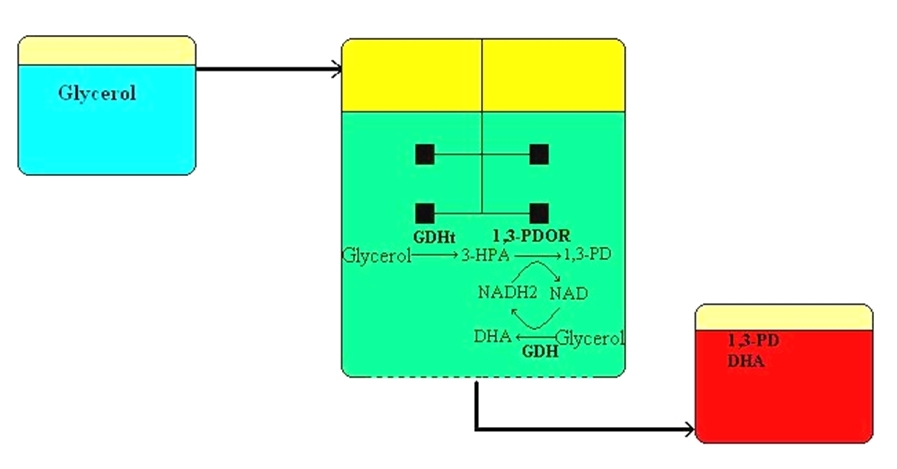
During the research, we put emphasis on the enzyme
production, since they are not available commercially. Beside the
1,3-propanediol producer strains existing in nature we started developing our
own recombinant E. coli for more effective enzyme production purposes.
Several questions have already been answered in our research (for
example: Can such a system be operated? Yes, it can. Can enzymes be used repeatedly, over several cycles? Yes, they can. And so on.). However, several important questions
are still open. For example: Can batch operation of this system be converted into
continuous mode? What are the common optimal pH and temperature
values and enzyme ratio of the three key enzymes? Is it possible to scale up the
process? And so on.
The first milestone of the lactic acid research was laid, and our results were summarized in Kata Hetényi's PhD dissertation (i.e. thesis). During this first step, we identified the agricultural raw materials and associated nutrient supplementation that mesophilic microbes required for their effective L-lactic acid production. We also examined their technical parameters. Some thermotolerant producer strain have also been selected, but their further examination is necessary to overcome their product inhibition by lactic acid, their pH controlling challenges and their scale up. These researches are in process.
Methodology
For the enzymatic bioconversion of 1,3-propanediol a special Clostridium butyricum strain was applied. Its speciality is that while other 1,3-propandiol (and its key enzymes) producer have a B12 dependent glycerol-dehydratase (GDHt), which goes under suicide inactivation with its coenzyme, the GDHt of the applied C. butyricum is B12-independent. At the same time, this microbe needs strict anaerobic conditions (full air displace), which makes the enzyme production more difficult. As the majority of bacteria, this strain can also be best cultivated on complex media, which is technologically not advantageous because of its high and costly organic nitrogen-source content (e.g. tryptone, yeast extract) that remain mostly intact causing difficulties during enzyme recovery, and can contaminate the product. For this reason, we focus our efforts on the determination of the required minimal but sufficient components and their quantity.
The key enzymes are formed intracellularly (inside the cells), thus the
culture is sonicated after fermentation, and cell debris is removed by way of
centrifugation. In the resulting supernatant solution the whole
protein (and enzyme) content of the cells is present, and the key enzymes should be
purified from this protein matrix. We also examined to what extent should the
enzymes be purified to keep the process cost effective and maintain the required biocatalytic activity at the same time. The obtained enzymes are highly sensitive -
especially to oxygen -, thus stabilizing them is a crucial point for any
application. We have good results with covalent immobilization in this respect.
To immobilise the enzymes we use chitosan (produced from fishing wastes
(chitin)) for manufacturing polymer beads with glutaraldehyde, which binds
enzymes to the beads. After the immobilization of key enzymes in this way
– as our results demonstrated – the enzymes have significantly higher
stability.
It is an important part of this research to determine the activity of the enzymes. This task is quite difficult, even in the case of soluble enzymes, due to the complex background matrix. Development of an analytical process for activity measurement of immobilised enzymes has just been started.
From the beginning of the research, the enzyme source is the above-mentioned
C. butyricum, but this natural strain has its limitations (enzyme
recovery is limited, by-product formation problems, anaerobic demand etc.), thus
we started to clone the key enzymes into a recombinant bacterium. The anticipated
success of this method will give a new impetus for the examination of the enzymatic
system.
In the case of lactic acid, the applied methods were summarized in several publications, so we only touch them briefly: 1) we selected a Lactobacillus strain, and scaled up on a laboratory media 2) this was reproduced in real industrially applicable media, too (primarily on wheat floor basis, later on basis of sweet sorghum broth, and determined in both cases the nutrient supplementation with statistical experimental design), 3) then we solved the pH controlling problems 4) and finally we set up and applied a kinetic model for determination of the optimal inoculation time during starch hydrolysis (in wheat flour) to obtain a combined hydrolysis and fermentation technique.
Since we found on each raw materials that a minimal yeast extract demand is
essential – according to the Biorefinery concept – we examined the possibility
of the in house yeast extract production via yeast fermentation on
different wastes of sweet sorghum (i.e. cellulose or hemicellulose fraction of
bagasse). To decide whether sweet sorghum broth or pentose or hexose fraction
of the bagasse would be economically more feasible, we used flowsheeting for
technology simulation. This indicated that sweet sorghum broth is the most cost
effective way for an in house yeast extract production.
Results
During the enzymatic 1,3-propanediol production we have performed several successful glycerol-propanediol bioconversions in a membrane reactor (enzymes immobilized behind an ultrafilter membrane) in batch operation. By discharging the whole broth through the membrane and subsequently refilling the reactor with fresh glycerol solution, it was verified that even the soluble enzymes are capable of multi cycle usage. Since the enzyme solution was not purified, in these cases dihydroxyaceton (DHA) was phosphorylated and partly converted by the glycolytic enzymes into by-products (like butyric acid, acetic acid, lactic acid).
Expected impact and further research
We created patent submissions from both researches (1,3-propanediol and lactic acid P 05 00961 and P 09 00193, respectively) and two summa cum laude PhD dissertations with a summarized impact factor of 5.84 in the international publications. The research group is ready for technology transfer in both fields for potential investors, although both procedures have further area of development. For example, in the case of lactic acid, the literature offers several efficient fermentation technics on the laboratory scale, but they have not been scaled up or used in the industry as yet. In our pilot laboratory we can investigate the reason why they have not been widely implemented in the industry.
In the case of PD, we already have results in the field of enzyme stabilization and their simultaneous application, however, with further optimization we expect to improve cost-efficiency, and thus increase the competitiveness of our process.
Publications, references, links
Publications:
(5 outstanding publications from the last 5 years)
K. Hetényi, Á. Németh, B. Sevella. Investigation and modeling of lactic
acid fermentation on wheat starch via SSF. CHF and SHF technology. Periodica
Polytechnica Chemical Engineering 2011, 55(1), 11-16 (online)
K. Hetényi, Á. Németh, B. Sevella. Role of pH-regulation in lactic acid
fermentation: second steps in a process improvement. Chemical Engineering and
Processing: Process Intensification 2011, 50, 293-299 (online)
K. Hetényi, Á. Németh, B. Sevella. Use of sweet sorghum juice for
lactic acid fermentation: preliminary steps in a process optimization. Journal
of Chemical Technology and Biotechnology 2010, 85, 872-877 (online)
A. Balássy, Á. Németh, B. Sevella. Immobilized enzymes availability for
glycerol -1,3-propanediol bioconversion. Hungarian Journal of Industrial
Chemistry 2009, 37, 83-88(online)
Á. Németh, B. Sevella. Development of a New Bioprocess for Production of 1,3-propanediol I.: Modeling of Glycerol Bioconversion to 1,3-propanediol with Klebsiella pneumoniae Enzymes. Applied Biochemistry and Biotechnology 2008, 144, (1), 47-58 (online)
(5 earlier publications)
A. Balássy, Á. Németh, B. Sevella. In New Enzymatical Process for Anaerobic Utilization of Glycerol. ChemPor 2008 10th International Chemical and Biological Conference. Braga, Portugal, 4-6 September, 2008
K. Hetényi, Á. Németh, B. Sevella. Researches on renewable resources at BUTE ABFS F-Labor. Fifth Croatian Professional and Scientific Conference on Biotechnology with International Participation. Stubicke Toplice, 2007
Á. Németh, B. Sevella. Research on the utilization of biodiesel byproduct (in Hungarian). Magyar Kémiai Folyóirat 2007, 113, (2), 58-61. (online)
Á. Németh, B. Sevella. Forschungen für enzymatische Herstellungen der
industriellen wertvollen Glyzerin-Derivate. 17. Frühlingsakademie von Institute
für Ingenieurweiterbildung der Technische und Wirtschaftwissenschaftliche
Universitaet. Budapest, Balatonfüred, 6th May, 2005
Á. Németh, B. Kupcsulik, B. Sevella. 1,3-Propanediol oxidoreductase
production with Klebsiella pneumoniae DSM2026. World Journal of
Microbiology and Biotechnology 2003, 19, (7), 659-663 (online)
Links
Bioengineering Working Group of HAS
Lactic acid from sweet sorghum
E-Vegyérték-electronic lecture notes
Introduction of participants
|
|
 |
 |
 |
|
Dr Nyeste László |
Dr
Sevella Béla professor |
Dr
Pécs Miklós associate professor |
Dr
Németh Áron senior lecturer |
 |
 |
||
|
Farkas
Ferenc technician |
Vargyas Tamásné cleaning referee |

